Study of temperature and ligand flexibility effects on coordination polymer formation from cyclobutanetetracarboxylic acid†
Abstract
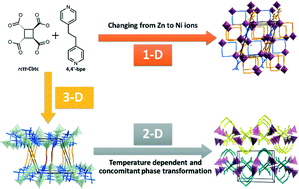
Three new coordination polymers based on the combination of rctt-1,2,3,4-cyclobutanetretracarboxylic acid (rctt-H4Cbtc) and trans-1,2-bis(4-pyridyl)ethane (4,4′-bpe) and Zn and Ni metal centers were synthesized under hydrothermal conditions and structurally characterized either by X-ray single crystal or powder diffraction: [Zn2(4,4′-bpe)(rctt-Cbtc)(OH2)]n·4H2O (1), [Zn(4,4′-bpe)0.5(rctt-Cbtc)0.5(OH2)]n (2) and [Ni2(4,4′-bpe)2(rctt-Cbtc)(OH2)6] (3). The crystal structure of 1 formed a 3-D coordination polymer. A similar building unit is found in 2 but it forms as a part of a polymeric 2-D array, where the bipyridine displays a gauche conformation in contrast to the anti conformation found for 1. In 3, the replacement of Zn with Ni as a node produces a novel supramolecular array based on a 1-D coordination polymer, which is constructed from chains bearing 4,4′-bpe in an anti configuration. A sequential hydrothermal transformation was observed between 1 and 2 in the range between 80 and 120 °C, respectively. An increase in time or temperature of reaction favored the formation of 2 below 140 °C. The solid phases of 1 and 2 are metastable above 140 °C. A novel unidentified phase is observed at 180 °C, starting from the same chemical precursors in a similar molar ratio. DFT calculations corroborate the experimental observations; compound 2 is a little more stable than 1. The energy lost when water leaves the Zn atom is partially compensated by the O atom of the rctt-Cbtc ligand and could induce the formation of compound 2.

 Please wait while we load your content...
Please wait while we load your content...