 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Template-free synthesis of β-Na0.33V2O5 microspheres as cathode materials for lithium-ion batteries
Qinguang
Tan†
a,
Qinyu
Zhu†
a,
Anqiang
Pan
*a,
Yaping
Wang
a,
Yan
Tang
a,
Xiaoping
Tan
a,
Shuquan
Liang
*a and
Guozhong
Cao
*b
aSchool of Materials Science & Engineering, Central South University, Changsha, Hunan 410083, China. E-mail: pananqiang@csu.edu.cn; lsq@mail.csu.edu.cn
bDepartment of Materials Science & Engineering, University of Washington, Seattle, 98195, USA. E-mail: gzcao@u.washington.edu
First published on 25th May 2015
Abstract
In this work, hierarchical three-dimensional (3D) β-Na0.33V2O5 microspheres have been synthesized for the first time by a simple solvothermal reaction with subsequent annealing treatment. The hierarchical microspheres are self-assembled from nanosheet subunits during the solvothermal process. Simultaneously, various hierarchical microspheres with different subunits can be produced by altering the solvothermal solution. After annealing in air, the solvothermal product can be converted into β-Na0.33V2O5 with a well-retained structure. As cathode materials for lithium ion batteries, the as-prepared 3D microspheres exhibit high capacity and good rate capability. The superior electrochemical performance is attributed to the unique three-dimensional hierarchical microstructures, which mimic the advantages of nano- and micro-structures.
1 Introduction
Recently, three-dimensional (3D) nanostructured materials have attracted considerable attention due to their superior physical and chemical properties.1–5 In particular, they are broadly studied as electrodes for lithium ion batteries (LIBs) because of their capability to maintain the structural integrity, high packing density and good lithium storage properties.6–9 Moreover, the characteristic hierarchical structures are believed to have better abilities to suppress agglomeration, facilitate electrolyte penetration and accommodate the volume change upon cycling.10–12 The nanosized building blocks could also effectively reduce the lithium diffusion distance, which could significantly improve the rate capability and capacity retention.10,13,14 To date, the hierarchical micro/nanostructures, such as porous frameworks,1,15–18 hollow structured microspheres13,19 and urchin-like microflowers,20,21 have been fabricated to investigate their electrochemical properties. However, the majority of reports are based on metal oxides with simple compositions. It is still a big challenge to fabricate hierarchical lithium/sodium transition metal oxides with complex compositions, such as LiMn2O4 and Na0.33V2O5, which significantly limits their applications.22–29Vanadium oxides and their derivatives have attracted research interest as alternative cathode materials for LIBs for decades, mainly because of their high specific capacities, low cost and abundant resources.16,29–37 Among the vanadium oxide derivatives, β-phase Na0.33V2O5 is believed to be a promising candidate due to its 3D tunneled crystal structure and high capacity.38–41 To date, low dimensional β-Na0.33V2O5 nanowires42,43 and mesoporous nanoflakes39 have been reported to have improved electrochemical performance. However, the synthesis of β-Na0.33V2O5 hierarchical structures is rarely reported.
Herein, we report the fabrication of β-Na0.33V2O5 hierarchical microspheres by a solvothermal approach with a subsequent annealing process in air. The solvothermally prepared microspheres are assembled from small nanosheets and the structures can be safely retained after calcination in air. As cathode materials for lithium ion batteries, the β-Na0.33V2O5 microspheres exhibit high capacity and good rate capability.
2 Experimental section
Materials preparation
V2O5 (0.36 g) and H2C2O4·2H2O in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were dissolved in 12 mL of distilled water under vigorous stirring at 60 °C for several hours until a clear blue solution was formed. Then, NaHCO3 with different molar ratios (Na
3 were dissolved in 12 mL of distilled water under vigorous stirring at 60 °C for several hours until a clear blue solution was formed. Then, NaHCO3 with different molar ratios (Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 1
6 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was separately added to the above-prepared solution under stirring for 20 minutes. After that, 60 mL of isobutanol was added to prepare the mixture solution, which was transferred into a sealed 100 mL Teflon container and treated in an electrical oven at 180 °C for 48 h. The obtained precursors were collected by centrifugation and washed twice with pure ethanol, followed by drying at 70 °C overnight. The as-prepared precursors were annealed in air at 400 °C for 3 h with a heating rate of 1 °C min−1. The as-synthesized products obtained with molar ratios of Na
3) was separately added to the above-prepared solution under stirring for 20 minutes. After that, 60 mL of isobutanol was added to prepare the mixture solution, which was transferred into a sealed 100 mL Teflon container and treated in an electrical oven at 180 °C for 48 h. The obtained precursors were collected by centrifugation and washed twice with pure ethanol, followed by drying at 70 °C overnight. The as-prepared precursors were annealed in air at 400 °C for 3 h with a heating rate of 1 °C min−1. The as-synthesized products obtained with molar ratios of Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 1
6 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were designated as NVO-1, NVO-2 and NVO-3, respectively.
3 were designated as NVO-1, NVO-2 and NVO-3, respectively.
Materials characterization
TG and DSC analyses were performed on a combined differential scanning calorimetry and thermogravimetric analysis instrument (Netzsch STA 449C, Germany). The crystallographic phases of all the products were investigated by powder X-ray diffraction (XRD, Rigaku D/max 2500) with Cu Kα (λ = 1.5406 Å) radiation. The morphologies of samples were examined by field-emission scanning electron microscopy (SEM, FEI Nova NanoSEM 230) and transmission electron microscopy (TEM, JEOL-JEM-2100F transmission electron microscope).Electrochemical measurements
The working cathode slurry was prepared by dispersing the β-Na0.33V2O5, acetylene black and poly(vinylidene fluoride) (PVDF) binder in N-methylpyrrolidone solution at a weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The slurry was painted on an aluminum foil and dried in a vacuum oven at 110 °C overnight prior to coin-cell assembly. A lithium foil was used as the current collector and reference electrode, and 1.0 M LiPF6 in ethyl carbonate/dimethyl carbonate (1
10. The slurry was painted on an aluminum foil and dried in a vacuum oven at 110 °C overnight prior to coin-cell assembly. A lithium foil was used as the current collector and reference electrode, and 1.0 M LiPF6 in ethyl carbonate/dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio) was used as the electrolyte. Cyclic voltammetry (CV) measurements were performed on an electrochemical workstation (CHI604E, China). The galvanostatic charge/discharge performance of the electrodes was evaluated at room temperature using a Land battery tester (Land CT 2001A, China). Electrochemical impedance spectrometry (EIS) was carried out on a ZAHNER-IM6ex electrochemical workstation in the frequency range of 100 KHz to 10 mHz.
1 v/v ratio) was used as the electrolyte. Cyclic voltammetry (CV) measurements were performed on an electrochemical workstation (CHI604E, China). The galvanostatic charge/discharge performance of the electrodes was evaluated at room temperature using a Land battery tester (Land CT 2001A, China). Electrochemical impedance spectrometry (EIS) was carried out on a ZAHNER-IM6ex electrochemical workstation in the frequency range of 100 KHz to 10 mHz.
3 Results and discussion
Fig. 1 shows the SEM images of the solvothermally prepared microsphere precursors at different magnifications. As shown in Fig. 1a and b, the solvothermal products obtained with the molar ratio of Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 are of spherical morphology, the diameter of which is about 4 μm. The microspheres are monodispersed. The higher magnification SEM image (Fig. 1c) further reveals the detailed structures of the microspheres, which are assembled from discrete nanosheets with a thickness of about 30 nm. The formation of hierarchical microspheres in the solvothermal process is attributed to the mixed solvent (isobutanol and water). The whole solvent can be considered as the accumulation of small water and isobutanol droplets, and the precursor subunits are formed within these droplets. When the subunits grow big enough, they will break the volume limitation of the small droplets and the subunits will merge together in order to reduce the surface energy to reach a more thermodynamically stable state. Therefore, the hierarchical microspheres will be formed. As shown in Fig. 2a and b, when NaHCO3 with a molar ratio of Na
4 are of spherical morphology, the diameter of which is about 4 μm. The microspheres are monodispersed. The higher magnification SEM image (Fig. 1c) further reveals the detailed structures of the microspheres, which are assembled from discrete nanosheets with a thickness of about 30 nm. The formation of hierarchical microspheres in the solvothermal process is attributed to the mixed solvent (isobutanol and water). The whole solvent can be considered as the accumulation of small water and isobutanol droplets, and the precursor subunits are formed within these droplets. When the subunits grow big enough, they will break the volume limitation of the small droplets and the subunits will merge together in order to reduce the surface energy to reach a more thermodynamically stable state. Therefore, the hierarchical microspheres will be formed. As shown in Fig. 2a and b, when NaHCO3 with a molar ratio of Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 was added to the solvothermal solution, nanosheet-structured microflowers were obtained after solvothermal treatment. The diameter of the microflowers was much smaller, ranging from 2 to 3 μm. As shown in Fig. 2c and d, urchin-like NVO-3 nano/microstructures could be produced when NaHCO3 with a higher molar ratio of Na
6 was added to the solvothermal solution, nanosheet-structured microflowers were obtained after solvothermal treatment. The diameter of the microflowers was much smaller, ranging from 2 to 3 μm. As shown in Fig. 2c and d, urchin-like NVO-3 nano/microstructures could be produced when NaHCO3 with a higher molar ratio of Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was employed. The urchin-like subunits were composed of nanobelts with a length of about 3 μm. Also, the urchin-like microspheres were the largest among the three solvothermally prepared samples. When a higher amount of NaHCO3 was added, the subunits of the hierarchical microstructures changed from nanosheets to nanobelts. The morphology change may be attributed to the higher concentration of Na+ ions, which may serve as the nuclei centers of the precursors. When more Na+ ions are provided in the solvothermal solution, more subunits start to grow and the species in the solution are depleted much faster, thus smaller subunits (nanobelts) are formed. This behavior is a little similar to that in the previous report on the synthesis of VO2 microstructures.20 The results demonstrated that hierarchical microstructures with different morphologies can be obtained by simply altering the amount of added NaHCO3.
3 was employed. The urchin-like subunits were composed of nanobelts with a length of about 3 μm. Also, the urchin-like microspheres were the largest among the three solvothermally prepared samples. When a higher amount of NaHCO3 was added, the subunits of the hierarchical microstructures changed from nanosheets to nanobelts. The morphology change may be attributed to the higher concentration of Na+ ions, which may serve as the nuclei centers of the precursors. When more Na+ ions are provided in the solvothermal solution, more subunits start to grow and the species in the solution are depleted much faster, thus smaller subunits (nanobelts) are formed. This behavior is a little similar to that in the previous report on the synthesis of VO2 microstructures.20 The results demonstrated that hierarchical microstructures with different morphologies can be obtained by simply altering the amount of added NaHCO3.
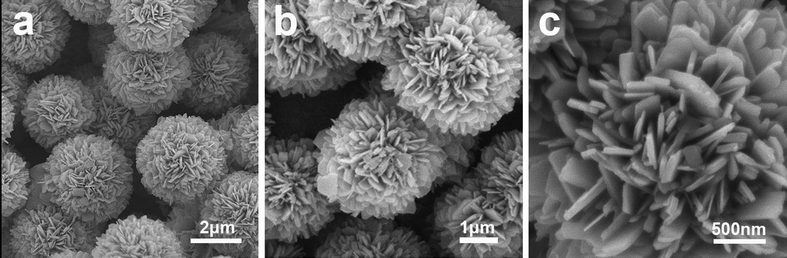 | ||
| Fig. 1 FESEM images (a, b, c) with different magnifications of the hierarchical microsphere precursor solvothermally prepared at 180 °C for 48 hours (NVO-1 precursor). | ||
 | ||
| Fig. 2 FESEM images of the hierarchical microflowers solvothermally prepared after changing the amount of NaHCO3: (a and b) NVO-2 precursor; (c and d) NVO-3 precursor. | ||
Fig. 3 shows the TG and DSC results of the precursor microspheres calcined in air using a heating rate of 10 °C min−1. The first weight loss below 250 °C is attributed to the evaporation of the physically absorbed and crystalline hydrate in the precursor. The main weight loss in the TG curve and the corresponding exothermic peak in the DSC curve at 366 °C are ascribed to the formation of β-Na0.33V2O5. The sharp endothermic peak in the DSC curve at 582 °C suggests the melting of β-Na0.33V2O5. After annealing in air, the precursor microspheres can be converted into β-Na0.33V2O5 with well-preserved morphologies. Fig. 4a shows the XRD pattern of the product (NVO-1) calcined in air at a relatively low temperature of 400 °C for 3 h, which can be indexed to a β-Na0.33V2O5 phase (space group: A2/m (12), JCPDS card 86-0120).38,39 A panoramic view of the product (Fig. 4b) demonstrates that the morphologies of the microspheres obtained from the solvothermal products after annealing in air are well preserved. The space between neighboring nanosheets is clearly detected (Fig. 4c). The nanosheet subunits shrunk to smaller nanosheets during the annealing process, which may be attributed to the recrystallization of the precursor when it was converted into β-Na0.33V2O5. The TEM image (see Fig. 4d) reveals that the microspheres are composed of nanosheet subunits and are of high porosity. The good porosity is beneficial for electrolyte penetration into the electrode materials. The hierarchical nature of the β-Na0.33V2O5 microspheres is further evaluated by nitrogen adsorption–desorption measurement and the results are shown in Fig. 5. The isothermal curve exhibits a typical χ type, indicating macroporous characteristics. The measured Brunauer–Emmett–Teller (BET) area of the β-Na0.33V2O5 microspheres is 12.5 m2 g−1. Barrett–Joyner–Halenda (BJH) calculations disclose that the pore size distribution is in the range of 3–20 nm, which is in good correspondence with the TEM image.
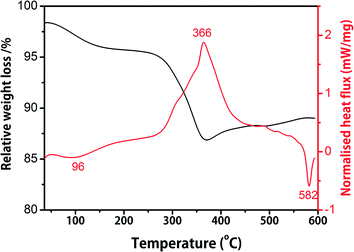 | ||
| Fig. 3 TG and DSC results of the as-obtained precursor after the solvothermal process. The heating rate was set to 10 °C min−1. | ||
 | ||
| Fig. 4 (a) XRD pattern, (b and c) FESEM images and (d) TEM image of the as-synthesized β-Na0.33V2O5 microspheres (NVO-1) obtained by annealing the precursor microspheres at 400 °C for 3 h. | ||
 | ||
| Fig. 5 (a) Nitrogen adsorption–desorption isotherm and (b) the corresponding pore size distribution of the β-Na0.33V2O5 microspheres. | ||
The β-Na0.33V2O5 microspheres were assembled into coin-cells and evaluated as cathode materials for LIBs. Fig. 6a shows the typical cyclic voltammetry (CV) curve of the β-Na0.33V2O5 microspheres at a scan rate of 0.1 mV s−1 between 1.5 and 4.0 V vs. Li/Li+. During the cathodic scan, five cathodic peaks located at 3.22, 2.88, 2.46, 2.20 and 1.88 V are observed, indicating the multiple-step lithium ion intercalation process.35–37Fig. 6b shows both the representative discharge–charge profiles of the β-Na0.33V2O5 electrode between 1.5 and 4.0 V at current densities of 50, 600 and 1000 mA g−1. The potentials for the five plateaus on the discharge profiles of the first cycle are highly consistent with the CV results. The β-Na0.33V2O5 microspheres exhibit high initial specific capacities of 249, 216, and 157 mA h g−1 at current densities of 50, 600 and 1000 mA g−1, respectively, indicating the good rate capability of the electrode materials. Fig. 6c shows the cycling performance of the β-Na0.33V2O5 microspheres at a current density of 1000 mA g−1. They exhibit an initial discharge capacity of 157 mA h g−1 and retain a specific discharge capacity of 111 mA g−1 after 35 cycles. The average capacity fading rate per cycle is 0.8%. The good cycling performance at high current density can be attributed to the novel three dimensional microstructures assembled from nanosheet building blocks. The hierarchical 3D microspheres can limit self-aggregation upon cycling. The large space between neighboring subunits can provide pathways for easy electrolyte penetration and the nanosized subunits can shorten lithium ion diffusion and electron transport distances, thus allowing the electrode to obtain good rate capability and cyclic stability. In order to study the transport kinetics of the electrochemical properties of the microspheres, the typical electrochemical impedance spectra of the electrodes were obtained and the Nyquist plots are shown in Fig. 6d. The impedance spectra consist of a depressed semicircle in the high-frequency region and a straight line in the low-frequency region. The semicircle is assigned to the formation of solid electrolyte interface (SEI) films and contact resistance, and the straight line is associated with the diffusion of Li+ in the electrode materials. In the equivalent circuit model (inset of Fig. 6d), R1 consists of the electrolyte resistance and ohmic resistances of cell components. R2 is the resistance of the SEI films while R3 is the charge transfer resistance of the electrochemical reaction. The value of R3 increases with cycling due to the increasing thickness of SEI films and irreversible phase transformation, which is commonly seen as a dominant effect on the capacity fading of vanadium based cathodes.44,45 As shown in the Nyquist plots, for the fresh electrode, the charge transfer resistance value is 121.6 Ω. When the electrode was evaluated for 35 cycles, the charge transfer resistance increases to 181.3 Ω, indicating a slight increase of charge transfer resistance but a lower increase rate compared with that of the mesoporous β-Na0.33V2O5 electrode.39 The small charge-transfer resistance can be attributed to the unique hierarchical structures, which shorten the diffusion pathways and improve the structure stability during the cycling processes. To further investigate the structure stability of β-Na0.33V2O5 electrodes, the morphology of the β-Na0.33V2O5 microspheres after the cycling processes was characterized by SEM. As shown in Fig. 7, after cycling at 1000 mA g−1 for 35 cycles, it is interesting to find that the hierarchical structures of the microspheres can be retained, which indicates the good structural stability of the hierarchical structures.
 | ||
| Fig. 7 Morphological analysis of the β-Na0.33V2O5 microspheres cycled for 35 cycles at a current density of 1000 mA g−1. | ||
Conclusions
In summary, 3D hierarchical β-Na0.33V2O5 microspheres have been successfully synthesized though a simple solvothermal method with a subsequent annealing process. The morphologies of the solvothermally prepared microstructures can be adjusted by changing the amount of added NaHCO3. The nanosheet-assembled microspheres can be converted into β-Na0.33V2O5 with good structural preservation after annealing. As cathode materials for LIBs, the β-Na0.33V2O5 microspheres exhibit high rate capability and enhanced cycling stability.Acknowledgements
This work was supported by the National High-tech R&D Program (863, grant no. 2013AA110106), the National Natural Science Foundation of China (no. 51374255 and 51302323), the Program for New Century Excellent Talents in University (NCET-13-0594), Research Fund for the Doctoral Program of Higher Education of China (no. 201301621200), the Natural Science Foundation of Hunan Province, China (14JJ3018), and the Lie-Ying and Sheng-Hua Program of Central South University.References
- Q. An, F. Lv, Q. Liu, C. Han, K. Zhao, J. Sheng, Q. Wei, M. Yan and L. Mai, Nano Lett., 2014, 14, 6250–6256 CrossRef CAS PubMed.
- J. Bai, X. Li, G. Liu, Y. Qian and S. Xiong, Adv. Funct. Mater., 2014, 24, 3012–3020 CrossRef CAS PubMed.
- J. S. Chen, D. Luan, C. M. Li, F. Y. Boey, S. Qiao and X. W. Lou, Chem. Commun., 2010, 46, 8252–8254 RSC.
- X. Fan, J. Shao, X. Xiao, L. Chen, X. Wang, S. Li and H. Ge, J. Mater. Chem. A, 2014, 2, 14641 CAS.
- C. Cheng, W. Ren and H. Zhang, Nano Energy, 2014, 5, 132–138 CrossRef CAS PubMed.
- Z. Fang, Q. Wang, X. Wang, F. Fan, C. Wang and X. Zhang, Mater. Res. Bull., 2013, 48, 4935–4941 CrossRef CAS PubMed.
- X. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604 CAS.
- D. Li, Q. Qin, X. Duan, J. Yang, W. Guo and W. Zheng, ACS Appl. Mater. Interfaces, 2013, 5, 9095–9100 CAS.
- L. Hu, H. Zhong, X. Zheng, Y. Huang, P. Zhang and Q. Chen, Sci. Rep., 2012, 2, 986 Search PubMed.
- L. Liu, Q. Fan, C. Sun, X. Gu, H. Li, F. Gao, Y. Chen and L. Dong, J. Power Sources, 2013, 221, 141–148 CrossRef CAS PubMed.
- X. W. Lou, C. Yuan and L. A. Archer, Adv. Mater., 2007, 19, 3328–3332 CrossRef CAS PubMed.
- X. W. Lou and L. A. Archer, Adv. Mater., 2008, 20, 1853–1858 CrossRef CAS PubMed.
- A. Pan, H. B. Wu, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 2226–2230 CrossRef CAS PubMed.
- C. Nethravathi, C. R. Rajamathi, M. Rajamathi, U. K. Gautam, X. Wang, D. Golberg and Y. Bando, ACS Appl. Mater. Interfaces, 2013, 5, 2708–2714 CAS.
- R. B. Rakhi, W. Chen, M. N. Hedhili, D. Cha and H. N. Alshareef, ACS Appl. Mater. Interfaces, 2014, 6, 4196–4206 CAS.
- C. Zhang, Z. Chen, Z. Guo and X. W. Lou, Energy Environ. Sci., 2013, 6, 974 CAS.
- L. Huang, X. Zhao, L. Zhang, L. Y. Shi, J. P. Zhang and D. S. Zhang, Nanoscale, 2015, 7, 2743–2749 RSC.
- S. Cai, D. Zhang, L. Shi, J. Xu, L. Zhang, L. Huang, H. Li and J. Zhang, Nanoscale, 2014, 6, 7346–7353 RSC.
- J. Zhu, Z. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Yan, Energy Environ. Sci., 2013, 6, 987 CAS.
- C. Niu, J. Meng, C. Han, K. Zhao, M. Yan and L. Mai, Nano Lett., 2014, 14, 2873–2878 CrossRef CAS PubMed.
- H. Li, D. Zhang, P. Maitarad, L. Shi, R. Gao, J. Zhang and W. Cao, Chem. Commun., 2012, 48, 10645–10647 RSC.
- K. Kim do, P. Muralidharan, H. W. Lee, R. Ruffo, Y. Yang, C. K. Chan, H. Peng, R. A. Huggins and Y. Cui, Nano Lett., 2008, 8, 3948–3952 CrossRef PubMed.
- S. Liang, T. Chen, A. Pan, D. Liu, Q. Zhu and G. Cao, ACS Appl. Mater. Interfaces, 2013, 5, 11913–11917 CAS.
- Y. Tang, D. Sun, H. Wang, X. Huang, H. Zhang, S. Liu and Y. Liu, RSC Adv., 2014, 4, 8328 RSC.
- S. Liang, J. Zhou, G. Fang, X. Li, A. Pan, J. Wu, Y. Tang and J. Liu, J. Alloys Compd., 2014, 583, 351–356 CrossRef CAS PubMed.
- H. He, G. Jin, H. Wang, X. Huang, Z. Chen, D. Sun and Y. Tang, J. Mater. Chem. A, 2014, 2, 3563 CAS.
- H. Song, Y. Liu, C. Zhang, C. Liu and G. Cao, J. Mater. Chem. A, 2015, 3, 3547–3558 CAS.
- S. Huang, X. L. Wang, Y. Lu, X. M. Jian, X. Y. Zhao, H. Tang, J. B. Cai, C. D. Gu and J. P. Tu, J. Alloys Compd., 2014, 584, 41–46 CrossRef CAS PubMed.
- H. Wang, S. Liu, Y. Ren, W. Wang and A. Tang, Energy Environ. Sci., 2012, 5, 6173 CAS.
- A. Q. Pan, H. B. Wu, L. Zhang and X. W. Lou, Energy Environ. Sci., 2013, 6, 1476 CAS.
- Y. L. Ding, Y. Wen, C. Wu, P. A. van Aken, J. Maier and Y. Yu, Nano Lett., 2015, 15, 1388–1394 CrossRef CAS PubMed.
- S. H. Bae, S. Lee, H. Koo, L. Lin, B. H. Jo, C. Park and Z. L. Wang, Adv. Mater., 2013, 25, 5098–5103 CrossRef CAS PubMed.
- S. Liang, J. Zhou, G. Fang, J. Liu, Y. Tang, X. Li and A. Pan, ACS Appl. Mater. Interfaces, 2013, 5, 8704–8709 CAS.
- C. Wang, Z. Guo, W. Shen, Q. Xu, H. Liu and Y. Wang, Adv. Funct. Mater., 2014, 24, 5511–5521 CrossRef CAS PubMed.
- D. Sun, G. Jin, H. Wang, X. Huang, Y. Ren, J. Jiang, H. He and Y. Tang, J. Mater. Chem. A, 2014, 2, 8009 CAS.
- L. Liang, Y. Xu, Y. Lei and H. Liu, Nanoscale, 2014, 6, 3536–3539 RSC.
- C. Han, Y. Pi, Q. An, L. Mai, J. Xie, X. Xu, L. Xu, Y. Zhao, C. Niu, A. M. Khan and X. He, Nano Lett., 2012, 12, 4668–4673 CrossRef CAS PubMed.
- R. Baddour-Hadjean, S. Bach, N. Emery and J. P. Pereira-Ramos, J. Mater. Chem., 2011, 21, 11296 RSC.
- S. Liang, J. Zhou, G. Fang, C. Zhang, J. Wu, Y. Tang and A. Pan, Electrochim. Acta, 2014, 130, 119–126 CrossRef CAS PubMed.
- G. Nagaraju, S. Sarkar, J. Dupont and S. Sampath, Solid State Ionics, 2012, 227, 30–38 CrossRef CAS PubMed.
- H. Liu, Y. Wang, L. Li, K. Wang, E. Hosono and H. Zhou, J. Mater. Chem., 2009, 19, 7885 RSC.
- Y. Xu, X. Han, L. Zheng, W. Yan and Y. Xie, J. Mater. Chem., 2011, 21, 14466 RSC.
- N. T. Hong Trang, N. Lingappan, I. Shakir and D. J. Kang, J. Power Sources, 2014, 251, 237–242 CrossRef CAS PubMed.
- S. Liang, J. Zhou, X. Zhang, Y. Tang, G. Fang, T. Chen and X. Tan, CrystEngComm, 2013, 15, 9869 RSC.
- H. Wang, K. Huang, C. Huang, S. Liu, Y. Ren and X. Huang, J. Power Sources, 2011, 196, 5645–5650 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2015 |

