Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Concomitant polymorphs of p-iso-propylcalix[4]arene†
Vincent J.
Smith
a,
Charl G.
Marais
a,
Kinga
Suwińska
b,
Janusz
Lipkowski
b,
Agnieszka
Szumna
c,
Catharine
Esterhuysen
a and
Leonard J.
Barbour
*a
aDepartment of Chemistry and Polymer Science, University of Stellenbosch, Matieland, 7602, South Africa. E-mail: ljb@sun.ac.za; Fax: +27 (0)21 808 3849; Tel: +27 (0)21 808 3335
bCardinal Stefan Wyszynski University in Warsaw, Faculty of Mathematical and Natural Sciences, Wojcickiego 1/3, 01-938 Warszawa, Poland
cInstitute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
First published on 11th March 2015
Abstract
Sublimation of p-iso-propylcalix[4]arene under reduced pressure results in the concomitant formation of two new polymorphs (forms IIP and IIIP). Both forms consist of interdigitated dimers of calixarene molecules, as does the known form IP. Despite similar inclusion behaviour to that of p-tert-butylcalix[4]arene, p-iso-propylcalix[4]arene does not appear to favour the formation of a transiently porous material.
Introduction
The extensive family of calix[n]arenes have a long history as host molecules in the field of inclusion chemistry.1 For example, the cone-shaped p-tert-butylcalix[4]arene (tBc) is well known for forming solvates with many different solvents. These solvates usually crystallise to adopt one of two types of host arrangements. One of these arrangements packs according to the space group P4/n, with a host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest ratio of 1
guest ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while the other crystallises in P4/nnc, with a host
1, while the other crystallises in P4/nnc, with a host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest ratio of 2
guest ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Both of these packing modes can be described in terms of a bilayer arrangement of alternating up-down calixarene molecules with guest solvent molecules inserted into the host clefts. Two different guest-free forms of tBc have been isolated using different methods of crystal growth. Ripmeester et al.2 crystallised tBc from heated tetradecane to produce the relatively close-packed (packing efficiency = 0.67) polymorph IB, in which the host molecules form mutually interdigitated dimers (i.e. one tert-butyl group of each molecule is inserted into the cavity of a facing host molecule). However, when tBc is sublimed at 280 °C under dynamic vacuum the host molecules once again adopt a bilayer-type arrangement with facing molecules slightly offset relative to one another (form IIB). The cavities of two facing molecules from adjacent layers combine to produce discrete voids of approximately 235 Å3 each, resulting in a relatively low packing efficiency of 0.59. At room temperature it is clear that all four tert-butyl groups of each calixarene are rotationally disordered whereas only one of the groups is disordered at 100 K. When form IIB is heated to 130 °C it undergoes a minor and reversible phase change to a high temperature form (IIIB), which has a similar bilayer packing arrangement to that of form IIB, except that adjacent bilayers become displaced laterally.3 Despite its apparent lack of permanent channels between lattice voids, form IIB possesses transient porosity, i.e. the crystals are able absorb a variety of solvents and gases into their discrete lattice voids, often in single-crystal to single-crystal (SC–SC) fashion. This was convincingly exemplified by a guest inclusion reaction involving the uptake of vinyl bromide with a concomitant SC–SC transformation of the host packing mode. Absorption of gases such as carbon dioxide, methane and acetylene does not result in rearrangement of the host molecules.3–5
1. Both of these packing modes can be described in terms of a bilayer arrangement of alternating up-down calixarene molecules with guest solvent molecules inserted into the host clefts. Two different guest-free forms of tBc have been isolated using different methods of crystal growth. Ripmeester et al.2 crystallised tBc from heated tetradecane to produce the relatively close-packed (packing efficiency = 0.67) polymorph IB, in which the host molecules form mutually interdigitated dimers (i.e. one tert-butyl group of each molecule is inserted into the cavity of a facing host molecule). However, when tBc is sublimed at 280 °C under dynamic vacuum the host molecules once again adopt a bilayer-type arrangement with facing molecules slightly offset relative to one another (form IIB). The cavities of two facing molecules from adjacent layers combine to produce discrete voids of approximately 235 Å3 each, resulting in a relatively low packing efficiency of 0.59. At room temperature it is clear that all four tert-butyl groups of each calixarene are rotationally disordered whereas only one of the groups is disordered at 100 K. When form IIB is heated to 130 °C it undergoes a minor and reversible phase change to a high temperature form (IIIB), which has a similar bilayer packing arrangement to that of form IIB, except that adjacent bilayers become displaced laterally.3 Despite its apparent lack of permanent channels between lattice voids, form IIB possesses transient porosity, i.e. the crystals are able absorb a variety of solvents and gases into their discrete lattice voids, often in single-crystal to single-crystal (SC–SC) fashion. This was convincingly exemplified by a guest inclusion reaction involving the uptake of vinyl bromide with a concomitant SC–SC transformation of the host packing mode. Absorption of gases such as carbon dioxide, methane and acetylene does not result in rearrangement of the host molecules.3–5
The fascinating solid-state inclusion properties of tBc prompted further studies aimed at tailoring the void space in apohost forms of close structural analogues. Some limited success was achieved by tetra-substitution of the tert-butyl groups with larger moieties such as tert-pentyl and tert-octyl groups. Although bilayer-type packing still occurred for the molecules with larger substituents, the more interesting guest-free offset face-to-face packing motif of IIB was not observed for these systems. It therefore appears that the host packing arrangement observed for IIB is highly sensitive to even slight addition of bulk to the upper rim of the calixarene.5,6
The present work was motivated by the observation (based on a search of the Cambridge Structural Database) that p-iso-propylcalix[4]arene (iPc, Scheme 1) exhibits host packing arrangements with a variety of guests that are highly reminiscent of that of tBc with the same guests.7,8 It was therefore hoped that, under the same crystal growth conditions, a slightly less bulky group on the upper rim would produce an apohost form that is isostructural to IIB, but with slightly more void space.
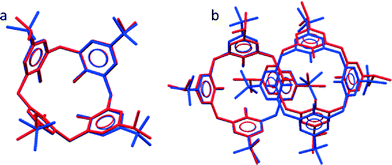
A previously published apohost form of iPc (refcode YARYAR, here designated as form IP) crystallises as interdigitated dimers and the structure is analogous to that of IB (refcode QIGBEN).7 An overlay of individual molecules of tBc and iPc shows that they adopt highly similar conformations, as expected (Fig. 1a). However, the degree of overlap between the dimers (Fig. 1b) of IB and IP is less precise, implying that the slightly different upper-rim moieties would significantly affect the overall extended periodicity of the two analogous structures relative to each other.
Results and discussion
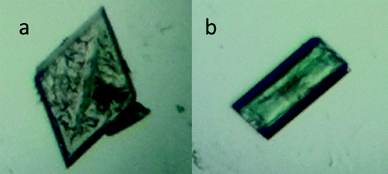
Perrin et al. had isolated apohost form IP from molten menthol at 65 °C.8 Instead, inspired by our previous work on tBc, we sublimed the as-synthesised iPc (i.e. the iPc⊂toluene solvate) under dynamic vacuum at 260 °C. Sublimation growth resulted in the formation of crystals with two distinct morphologies (Fig. 2) and single-crystal X-ray diffraction analysis confirmed that these are indeed concomitant polymorphs (designated as forms IIP and IIIP).For comparison, selected unit cell parameters for forms IP, IIP, IIIP and IB are reported in Table 1 (full crystallographic data provided in Table S1†). Forms IIP and IIIP also crystallise as interdigitated dimers while the hoped-for non-interdigitated phase (i.e. analogous to IIB) was not observed.
| IP | IIP | IIIP | IB | |
|---|---|---|---|---|
| Space group | P21/c | C2/c | P21/n | P21/c |
| a (Å) | 9.432(1) | 24.851(3) | 17.681(1) | 9.5878 |
| b (Å) | 15.349(2) | 9.412(1) | 9.525(1) | 30.5003 |
| c (Å) | 23.578(3) | 29.214(4) | 19.984(2) | 13.5412 |
| β (°) | 98.751(2) | 103.211(2) | 98.363(2) | 109.852 |
| D calc (g cm−3) | 1.167 | 1.182 | 1.184 | 1.47 |
| Temp. (K) | 100 | 100 | 100 | 173 |
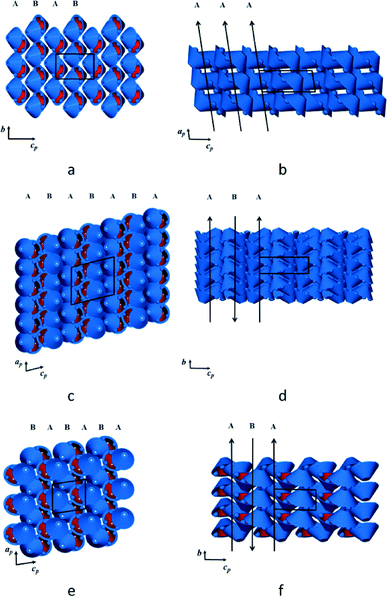
In the crystal structure of form IP the interdigitated dimers are stacked in columns parallel to [100]. In turn, these columns form layers parallel to (110) with alternating layers displaced laterally by 7.7 Å (Fig. 3a). The individual dimers (i.e. the mean plane through the eight iso-propyl groups) are inclined by approximately 32° relative to the bc plane and all the columns along a have the same orientation, as shown in Fig. 3b. In form IIP the dimers are stacked in columns parallel to [010] (Fig. 3c), which are arranged in layers parallel to [110] that stack along c. Consecutive layers have anti-parallel molecular orientations and are displaced laterally by approximately 5.0 Å (Fig. 3d). The dimers are inclined by about 6° relative to the ac plane. In form IIIP the dimers also stack in columns parallel to [010], with layers parallel to [110] and stacked along c (Fig. 3e). Alternate layers are displaced laterally by approximately 8.8 Å, with consecutive layers having an anti-parallel arrangement (Fig. 3f) and a molecular inclination angle of about 23° relative to the ac plane. It is noteworthy that the calculated densities of IP, IIP and IIIP differ by less than 1%.
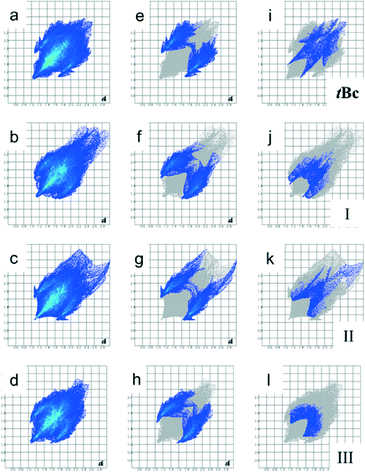
Hirshfeld surface analysis of the iPc polymorphs and of form IB of tBc was carried out using the program CrystalExplorer.9 The Hirshfeld surfaces were mapped using dnorm, which is a normalised contact that takes into account the van der Waals radius of each atom. Although the 2D fingerprint plots for the form IB apohost and the three polymorphs of iPc are quite different, they show strikingly similar features (Fig. 4a–d). The iPc polymorphs and form IB experience multiple C–H⋯π interactions, as evidenced by the ‘saw-tooth’ shapes on either side of the diagonal de = di (Fig. 4e–h). These interactions result from the insertion of a single tert-butyl or iso-propyl moiety into its partner's cavity by the two calixarenes comprising a dimer.
The C–H⋯π interactions for form IB contribute 20.3% to the total area of the Hirshfeld surface while those of the iPc polymorphs contribute 23.4%, 24.1% and 23.2% for forms IP, IIP and IIIP, respectively. The O⋯H(O) interactions, shown as short broad spikes directed towards the lower left corner of each plot, are due to the intramolecular hydrogen bonded ring formed by the hydroxyl groups on the lower rim of each calixarene (Fig. 4i–l). Interestingly, form IB also experiences weak O⋯H(C) interactions that are not present in the fingerprint plots of the iPc polymorphs – these result from interactions between hydrogen atoms belonging to tert-butyl moieties and the hydroxyl groups of neighbouring calixarenes. The total contributions of the O⋯H(O) (as well as O⋯H(C) interactions for form IB) to the Hirshfeld surfaces are 5.9, 6.3, 6.0 and 6.2% for forms IB, IP, IIP and IIIP, respectively.
Melting temperatures and enthalpies (Ton, Tpeak, ΔHfus and ΔHdes) were determined for the three polymorphs of iPc and iPc⊂toluene using differential scanning calorimetry (DSC) and these values are reported in Table 2. DSC thermograms for the phases IP, IIP and IIIP are shown in Fig. 5; each thermogram displays a single thermal event corresponding to the melt endotherm of each phase.
| Phase | T on (°C) | T Peak (°C) | ΔHfus (J g−1) | ΔHdes (J g−1) |
|---|---|---|---|---|
| Desolvation | 133.1 | 159.3 | — | 53.4 |
| Melt | 293.5 | 295.2 | 76.4 | — |
| IP | 294.3 | 296.1 | 75.3 | — |
| IIP | 289.9 | 292.4 | 62.1 | — |
| IIIP | 277.3 | 284.8 | 37.1 | — |
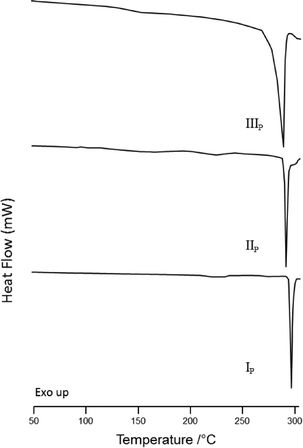 | ||
| Fig. 5 DSC traces of forms IP (bottom), IIP (middle) and IIIP (top) heated at 10 K min−1 in the range 25 to 315 °C. | ||
The iPc⊂toluene solvate (Fig. S1†) undergoes two thermal events that are related to desolvation and subsequent melting of the apohost. The observed melting point of the desolvated material corresponds to the melting point of form IP (Table 2). From the thermal data for the three apohost forms of iPc it appears that the highest melting phase (form IP) also has the highest heat of fusion. Indeed, the heats of fusion can be arranged in the order ΔHfusI > ΔHfusII > ΔHfusIII. Furthermore, we believe that the higher melting phase IP is also the thermodynamically most stable of the three forms since it can be crystallized from a saturated solution. According to the heat of fusion rule proposed by Burger et al. the three forms are monotropes (or monotropically related to one another).10
In order to understand the relationships between forms IP, IIP and IIIP, temperature-cycled DSC (TC-DSC) experiments were carried out by heating each sample in the range 25 to 315 °C, followed by cooling to 25 °C and then reheating to 315 °C. The TC-DSC experiments were carried out at different heating rates, ranging from 10 to 20 K min−1 at 5 K min−1 increments (Fig. S2†). Since the toluene solvate was used to generate the new phases IIP and IIIP in the sublimation experiment, it was also used as the starting phase for the TC-DSC experiments. The first TC-DSC experiment, which was carried out at a scanning rate of 10 K min−1 resulted in form IIIP, while a scanning rate of 15 K min−1 yielded a mixture of phases IIP and IIIP. From the ratio of the corresponding enthalpies we determined that more of form IIIP was produced than of form IIP (Fig. S3†). A rate of 20 K min−1 also resulted in a mixture of forms IIP and IIIP, but in this case the ratios were more evenly distributed. Powder X-ray diffraction (PXRD) was used to characterise the pure phases (Fig. 6) and also to confirm the identity of the phases obtained from DSC (calculated PXRD profiles Fig. S4†). The TC-DSC experiments imply that the cooling rate influences which phases are produced, and that forms IIP and IIIP are kinetic products, whereas IP is the thermodynamic product.
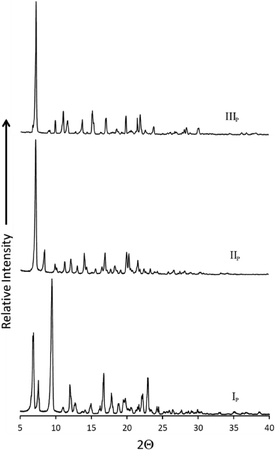 | ||
| Fig. 6 Experimental powder X-ray diffraction patterns of forms IP (bottom), IIP (middle) and IIIP (top). | ||
Conclusions
We have described the concomitant isolation and characterisation of two new polymorphs (IIP and IIIP) of iPc grown by sublimation. Similar to the known form IP, the two new forms consist of interdigitated calixarene dimers. Thermoanalytical measurements of all three forms imply that none of them interconvert to one another prior to their melting. Moreover, the higher melting form IP has the highest enthalpy of fusion and is most likely the thermodynamically stable form since it can be crystallised from solution. Forms IIP and IIIP appear to be kinetic forms since they can be isolated upon rapid cooling of the melt. We conclude that forms IP, IIP and IIIP are monotropes while IIP and IIIP can more specifically be considered kinetic monotropes of form IP. A temperature gradient within the glass oven used for the sublimation experiments could explain the origin of the concomitant forms since IIP and IIIP can be generated based on different cooling rates that only differ marginally.The original objective of this work was to establish whether iPc could fully mimic the highly interesting transient porosity of tBc. Unfortunately iPc does not appear to form an apohost form that is analogous to IIB. Although only one interdigitated form of pure tBc apohost is known (form IB), iPc yields three such forms as monotropic polymorphs of one another. This study demonstrates that, despite several marked similarities in their inclusion behaviour, tBc and iPc show very different packing trends in the manifestations of their apohost forms.
Acknowledgements
LJB, CE and VJS thank the National Research Foundation (NRF) of South Africa and Stellenbosch University for financial support.Notes and references
- C. D. Gutsche, Calixarenes Revisited, Royal Society of Chemistry, Cambridge, 1998 RSC; Calixarenes, ed. Z. Asfari, V. Bohmer, J. M. Harrowfield and J. Vicens, Kluwer, Dordrecht, 2001 RSC; G. D. Enright, K. A. Udachin and J. A. Ripmeester, Chem. Commun., 2004, 1360 RSC.
- E. B. Brouwer, G. D. Enright, K. A. Udachin, S. Lang, K. J. Ooms, P. A. Halchuk and J. A. Ripmeester, Chem. Commun., 2003, 1416 RSC.
- J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000 CrossRef CAS PubMed; J. L. Atwood, L. J. Barbour and A. Jerga, Chem. Commun., 2002, 2952 RSC; J. A. Atwood, L. J. Barbour, G. O. Lloyd and P. K. Thallapally, Chem. Commun., 2004, 922 RSC.
- L. J. Barbour, Chem. Commun., 2006, 1163 RSC; P. K. Thallapally, T. B. Wirsig, L. J. Barbour and J. L. Atwood, Chem. Commun., 2005, 51 Search PubMed; J. L. Atwood, L. J. Barbour and A. Jerga, Angew. Chem., Int. Ed., 2004, 43, 2948 CrossRef CAS PubMed; P. K. Thallapally, K. A. Kirby and J. L. Atwood, New J. Chem., 2007, 31, 628 RSC.
- P. K. Thallapally, T. B. Wirsig, L. J. Barbour and J. L. Atwood, Chem. Commun., 2005, 4420 RSC; P. K. Thallapally, L. Dobrańska, T. R. Gingrich, T. B. Wirsig, L. J. Barbour and J. L. Atwood, Angew. Chem., Int. Ed., 2006, 45, 6506 CrossRef CAS PubMed; S. J. Dalgarno, P. K. Thallapally, L. J. Barbour and J. L. Atwood, Chem. Soc. Rev., 2007, 36, 236 RSC.
- P. K. Thallapally, G. O. Lloyd, T. B. Wirsig, M. W. Bredenkamp, J. L. Atwood and L. J. Barbour, Chem. Commun., 2005, 5272 RSC; J. L. Atwood, L. J. Barbour and A. Jerga, Science, 2002, 296, 2367 CrossRef CAS PubMed.
- Cambridge Structural Database and Cambridge Structural Database System, Version 5.32, November 2011, Cambridge Crystallographic Data Centre, University Chemical Laboratory, Cambridge England Search PubMed.
- M. Perrin, F. Gharnati, D. Oehler, R. Perrin and S. Lecocq, J. Inclusion Phenom. Mol. Recognit. Chem., 1992, 14, 257 CrossRef CAS.
- S. K. Wolf, D. J. Grimwood, J. J. McKinnon, D. Jayatilaka and M. A. Spackman, CrystalExplorer 2.1, University of Western Australia, Perth, Australia, 2007 (http://hirshfeldsurface.net/CrystalExplorer/) Search PubMed.
- J. Bernstein, Polymorphism in Molecular Crystals, Oxford University Press, 2002 Search PubMed; A. Burger, Acta Pharm. Technol., Suppl., 1979, 7, 107 Search PubMed; A. Burger and R. Ramberger, Microchim. Acta, 1979, 2, 259 CrossRef CAS; A. Burger, Acta Pharm. Technol., 1982, 28, 17 Search PubMed; D. Giron, J. Therm. Anal. Calorim., 2001, 64, 37 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1048933–1048935. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ce00362h |
| This journal is © The Royal Society of Chemistry 2015 |