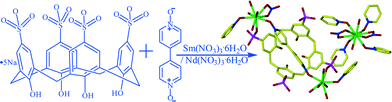Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel mixed 1D–2D lanthanide coordination polymers based on p-sulfonatocalix[4]arene and 4,4′-bipyridine-N,N′-dioxide where p-sulfonatocalix[4]arene acts as a guest†
Ahmad
Husain
and
Clive L.
Oliver
*
Centre for Supramolecular Chemistry Research, Department of Chemistry, University of Cape Town, Rondebosch, 7701, South Africa. E-mail: Clive.Oliver@uct.ac.za; Fax: +27 21 6505195; Tel: +27 21 6503830
First published on 16th March 2015
Abstract
Two novel mixed 1D–2D coordination polymers based on 2D [Ln(4,4′-bpdo)2(NO3)2(H2O)2]n+ sheets and 1D [Ln2(4,4′-bpdo)2(C4AS)(NO3)(H2O)9]n chains (Ln = Sm for 1 and Ln = Nd for 2) sustained by π⋯π interactions and lattice water facilitated hydrogen bonds have been established. The chains are arranged such that the p-sulfonatocalix[4]arene anions occupy the cavities of the 2D coordination polymer, and so act as guests rather than as hosts, which is their more usual role.
Introduction
Supramolecular chemistry of calixarenes offers a convenient approach to producing functional materials by directional and reversible non-covalent interactions,1,2 with special attention to cation⋯π, C–H, and π⋯π interactions3,4 including hydrogen bonds,5,6 host–guest recognition,1 donor–acceptor interactions,7,8 and metal–ligand interactions.9 Owing to the π-electron-rich hydrophobic cavity and the robust hydrophilic nature of the upper and lower rims,10–12 calixarenes have drawn much attention in the burgeoning area of supramolecular chemistry13 and coordination chemistry14,15 due to its host enwrapping ability of small molecules and as a ligands, developing coordination complexes or polymers with various metal ions.16–20 Specifically, p-sulfonatocalix[4]arenes with its pre-organized conical configuration and binding properties makes it an excellent model for molecular recognition and sensing, crystal engineering, catalysis, enzyme assays, and biological/medicinal chemistry.6,21–24Crowley and co-workers demonstrated that water-soluble p-sulfonatocalix[4]arene can bind lysine-rich cytochrome-c25 and lysozyme,26 forming a stable protein–calixarene complex, where p-sulfonatocalix[4]arene plays an important role in generating assemblies and promoting crystallization. Recently, Liu et al. also reported a supramolecular strategy to directly assemble the small molecular antipsychotic drug chlorpromazine into nanostructures, induced by p-sulfonatocalix[4]arene.8 Liu and co-workers showed strong host–guest interaction between p-sulfonatocalix[4]arenes and a viologen that lead to a high degree of polymerization and viscosity.27 Ling and co-workers established the self-assembly of p-sulfonatocalix[4]arene integrating various ionic liquid based symmetrical and unsymmetrical constituents in the presence of lanthanides and phosphonium cations.28,29 A novel strategy for the construction of supramolecular assemblies have also been proposed by Liu et al. for the complexation with p-sulfonatocalix[n]arenes to promote the aggregation of aromatic or amphiphilic molecules by lowering the critical aggregation concentration, enhancing the aggregate stability, and regulating the degree of order in the aggregates.30Since lanthanide ions have an extraordinary affinity for hard pyridyl-N-oxide donor groups, 4,4′-bipyridine-N,N′-dioxide (4,4′-bpdo) is an excellent candidate to construct 1D, 2D and 3D lanthanide metal coordination polymers. The relatively less sterically challenging nature, and orientation of the lone pairs on the pyridyl-N-oxide O atoms, results in a reasonably high flexibility apropos its coordination behaviour leading to structural diversity. Very recently, a channel supramolecular framework based on p-sulfonatocalix[4]arene nanocapsule using 4,4′-bipyridine-N,N′-dioxide and 2,2′-bipyridine-N,N′-dioxide together with Cu has been demonstrated by Zhang et al.31 In this structure a 2D framework constructed from Cu and 4,4′-bipyridine-N,N′-dioxide acts as a host for p-sulfonatocalix[4]arene capsules. Zhang et al. also reported the guest inducing of p-sulfonatocalix[4]arene into three-dimensional capsule architecture based on terbium/europium(III) and pyrazine-N,N′-dioxide assembling in to mixed A–B double layered framework.32 As part of our ongoing investigation of supramolecular assemblies based on the inclusion phenomena of p-sulfonatocalix[4]arene,33 we report here a novel mixed 1D–2D network based on p-sulfonatocalix[4]arene and 4,4′-bipyridine-N,N′-dioxide with Sm(III) and Nd(III). As was the case in the Zhang et al.31 structure the well-known host p-sulfonatocalix[4]arene acts as guest for a 2D coordination polymer, a reversal of its usual role.
Materials and physical measurements
All chemicals were of reagent grade, purchased from commercial sources, and used without further purification.Hot-stage microscopy was performed on a Nikon SMZ-10 stereoscopic microscope fitted with a Linkam THMS600 hot stage and a Linkam TP92 control unit. Samples were placed under silicone oil on a cover slip and heated at 10 °C min−1. Thermal events were monitored with a Sony Digital Hyper HAD colour video camera and captured using the Soft Imaging System program analysis.
Thermogravimetric analysis (TGA) measurements was performed at a heating rate of 10 °C min−1 in the temperature range 25–500 °C, under a dry nitrogen flow of 60 mL min−1 on a TGA Q500 instrument. About 3 mg of sample was placed in an open aluminium crucible.
Differential scanning calorimetry (DSC) measurement was performed at a heating rate of 10 °C min−1 in the temperature range 25–400 °C, under a dry nitrogen flow of 50 mL min−1 on a DSC Q200 instrument. About 2 mg of sample was placed in an aluminium pan with a lid. A sealed and empty pan was used as a reference.
Powder X-ray diffraction (PXRD) measurements were performed on a Bruker D8 Advance X-ray diffractometer in the 4–40° 2θ range using a 0.01° step size per second and X-rays generated at 30 kV and 40 mA.
Preparation of 1 as {[Sm(4,4′-bpdo)2(NO3)2(H2O)2]n}{[Sm2(4,4′-bpdo)2(C4AS)(NO3)(H2O)9]n}·4,4′-bpdo·NO3·12H2O
A mixture of Sm(NO3)3·6H2O (44.5 mg, 0.1 mmol), pentasodium p-sulfonatocalix[4]arene (120 mg, 0.1 mmol) and 4,4′-bipyridine-N,N′-dioxide, (20 mg, 0.1 mmol) with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (pH ~5) was dissolved in 3 ml hot water to yield a light yellow solution. Yellow block shaped crystals of compound 1 were obtained after volatilizing at 40 °C for a few days.
1 (pH ~5) was dissolved in 3 ml hot water to yield a light yellow solution. Yellow block shaped crystals of compound 1 were obtained after volatilizing at 40 °C for a few days.
Preparation of 2 as {[Nd(4,4′-bpdo)2(NO3)2(H2O)2]n}{[Nd2(4,4′-bpdo)2(C4AS)(NO3)(H2O)9]n}·4,4′-bpdo·NO3·12H2O
Compound 2 was obtained by similar procedure as for 1 except that Nd(NO3)3·6H2O was used instead of samarium nitrate.Single crystal X-ray diffraction analysis and structure determination
Suitable, apparently single crystals of 1 and 2 were selected and mounted on a cryoloop in oil. Data collection was carried out on a Bruker DUO APEX II CCD diffractometer using graphite monochromated Mo Kα (λ = 0.71073 Å) radiation with the crystal cooled to 100(2) K using an Oxford Cryostream-700. CELL_NOW identified 1 and 2 as 3- and 2-component non-merohedral twins, respectively.27 Data reduction and cell refinement were performed using SAINT-Plus.34 The X-ray diffraction data were corrected for Lorentz-polarization factor and scaled for absorption effects by using TWINABS.35 The structure was solved by direct methods, implemented in SHELXS-97.36 Refinement procedure by full-matrix least-squares method, based on F2 values against all reflections was performed by SHELXL-2014/7 using an ‘HKLF 5’ data format incorporating the data from all the twin domains,35 including anisotropic displacement parameters for all non-H atoms. The twin domains refined to values of (0.654(2), 0.1987(16), 0.1469(15)) for 1 and to (0.5075(6), 0.4925(6)) for 2, respectively. Although we were not able to locate all the hydrogen atoms for the disordered lattice water molecules, the contribution of hydrogen atoms are included in the SFAC instruction in the SHELXL-2014/7 refinement.CCDC 1035921 and 1035922 contains the supplementary crystallographic data for this paper.
Results and discussion
Compound 1 and 2 crystallized from a hot aqueous solution of pentasodium p-sulfonatocalix[4]arene (Na5C4AS) salt, 4,4′-bipyridine-N,N′-dioxide (4,4′-bpdo) and metal (Sm (1) and Nd(2)) nitrate hexahydrate (Scheme 1).Single crystal X-ray structure analyses reveal that complexes 1 and 2 are isostructural and crystallize in centrosymmetric space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , so 1 was chosen to represent the structural discussion. Crystallographic data are listed in Table 1.
, so 1 was chosen to represent the structural discussion. Crystallographic data are listed in Table 1.
| Empirical formula | C78H105N14O61S4Sm3 (1) | C78H105N14O61S4Nd3 (2) |
|---|---|---|
| Formula weight | 2793.95 | 2774.61 |
| Temperature (K) | 173(2) | 173(2) |
| λ (Å) | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Unit cell dimensions (Å), (°) | a = 15.9282(12), α = 116.84(2) | a = 15.9870(15), α = 116.97(2) |
| b = 18.6918(15), β = 91.34(2) | b = 18.7220(18), β = 91.38(2) | |
| c = 19.2219(15), γ = 90.92(2) | c = 19.3040(19), γ = 90.88(2) | |
| Volume (Å3) | 5102.3(7) | 5145.7(9) |
| Z | 2 | 2 |
| ρ (g cm−3) | 1.810 | 1.783 |
| μ (mm−1) | 1.903 | 1.688 |
| F 000 | 2792 | 2780 |
| Crystal size (mm3) | 0.55 × 0.24 × 0.20 | 0.52 × 0.27 × 0.12 |
| θ (°) | 1.18–27.00 | 1.18–28.44 |
| Miller index ranges | −20 ≤ h ≤ 20, −23 ≤ k ≤ 21, 0 ≤ l ≤ 24 | −21 ≤ h ≤ 21, −25 ≤ k ≤ 22, 0 ≤ l ≤ 25 |
| Reflections collected | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 697 697 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 582 582 |
| Independent reflections | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868 868 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 582 582 |
| Completeness to θmax (%) | 100 | 100 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868/1336/1519 868/1336/1519 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 582/1283/1503 582/1283/1503 |
| Goodness-of-fit on F2 | 1.053 | 1.033 |
| Final R indices [I > 2σ(I)] | R 1 = 0.0372, wR2 = 0.1070 | R 1 = 0.0471, wR2 = 0.1034 |
| R indices (all data) | R 1 = 0.0416, wR2 = 0.1101 | R 1 = 0.0737, wR2 = 0.1142 |
| Largest diff. peak and hole (e Å−3) | 1.229 and −0.979 | 1.291 and −0.882 |
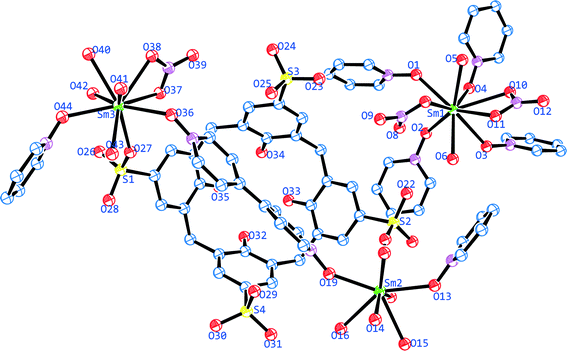
The asymmetric unit consist of two crystallographically independent coordination complex components (Fig. 1), one lattice 4,4′-bipyridine-N,N′-dioxide molecule, one lattice nitrate anion and twelve lattice water molecules. Component one, [Sm2(4,4′-bpdo)2(C4AS)(NO3)(H2O)9] is composed of one p-sulfonatocalix[4]arene moiety, one and two half 4,4′-bpdo molecules bridging Sm2 and Sm3 and so forms a zigzag 1-dimensional polymeric chain in the extended structure which propagates along the [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 02] direction. Component two, [Sm(4,4′-bpdo)2(NO3)2(H2O)2]+ is composed of Sm1 bonded to four half (bridging) 4,4′-bpdo ligands along with two nitrate anions in a bidentate coordination fashion, forming a 2-dimensional layer in the extended structure. The rest of the coordination sphere for the Sm1 cation is completed by two aqua ligands. All four 4,4′-bpdo ligands responsible for extending the 2D network are situated about inversion centres.
02] direction. Component two, [Sm(4,4′-bpdo)2(NO3)2(H2O)2]+ is composed of Sm1 bonded to four half (bridging) 4,4′-bpdo ligands along with two nitrate anions in a bidentate coordination fashion, forming a 2-dimensional layer in the extended structure. The rest of the coordination sphere for the Sm1 cation is completed by two aqua ligands. All four 4,4′-bpdo ligands responsible for extending the 2D network are situated about inversion centres.
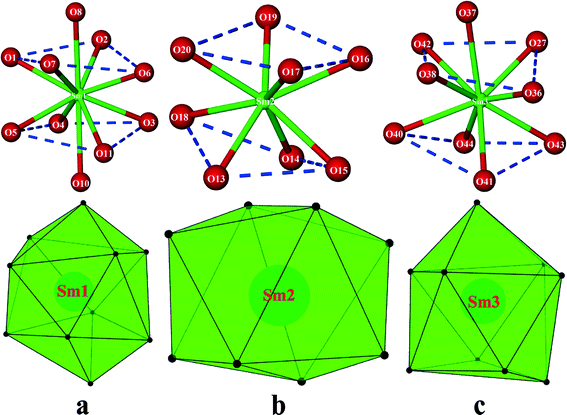
All the three crystallographically unique Sm(III) cations display different coordination geometries. The skeletal structure around Sm1 is a distorted bicapped square antiprism (Fig. 2a) with the O8 and O10 atoms from the nitrate anions at the bicapped positions, with the bond angle O10–Sm1–O8 being 160.42(10)°, which are at relatively longer distances (Tables S1 & S2†) than other O atoms from the Sm1 cation. The Sm2 cation is eight coordinated in a square antiprismatic fashion by eight O atoms (Fig. 2b) in which the set of atoms O16, O17, O19, O20 with a torsion angle of 12.76° and the set of atom O13, O14, O15, O18 with a torsion angle of 24.75° form two approximate square (top and bottom) planes, which are almost parallel to each other. The nine-coordinate Sm3 cation adopts a monocapped square antiprismatic geometry (Fig. 2c), having O37 from a ligated nitrate anion at the capped position. The Sm–O bond distances are in the 2.304(3)–2.675(3) Å range, which compares well with literature values.31,32,37,38 Selected bond lengths and bond angles are given in Tables S1 & S2.†
The Sm1 cation and four half 4,4′-bipyridine-N,N′-dioxide ligands form a 2D layered network based on a (4, 4) topology exhibiting bowl-shaped cavities which encapsulate the p-sulfonatocalix[4]arene anions (Fig. 3b). This is similar to the Zhang et al. structure,31 except that in this case neighbouring p-sulfonatocalix[4]arene entities, form a 1D polymer via coordination bonds. Even though the p-sulfonatocalix[4]arene is a ligand in forming the 1D polymer we take the view that ligation does not preclude the interaction between the 1D polymer and the 2D polymer from being described as a host–guest interaction where the 2D polymer forms the host cavity and p-sulfonatocalix[4]arene is the guest entity.39–41 In the crystal structure, the four Sm atoms and four 4,4′-bpdo ligands build up a bevel square shaped cavity with Sm⋯Sm distances of 13.410 and 13.668 Å. The torsion angles between the planes formed by opposite 4,4′-bpdo ligands of this framework are 31.40° and 65.57° while the torsion angle between the planes of the adjacent Sm atoms is 42.44° providing conical shape cavity. Similar motifs based on 4,4′-bpdo and 2,2′-bpdo with Cu(II) have also been observed by Zhang et al.,31 however this arrangement is completely different from that of Zhang et al. All four Cu atoms in the Zhang et al.31 motifs were almost co-planar with the O–Cu–O angle of 165 and 179.3°, whilst in our case the four metal ions are not co-planar with the O–Sm–O angles being 142.8° and 73.4°, probably to accommodate the coordinated nitrate anion in coordination sphere that finally lead to a conical cavity. This is further responsible for the slantwise long range weak π⋯π interactions.
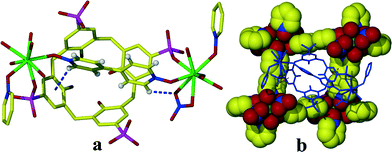 | ||
| Fig. 3 (a) 4,4′-bpdo ‘perched’ in the cavity of C4AS via C–H⋯π and C–H⋯O interactions. (b) C4AS residing in the cavity formed by [Sm(4,4′-bpdo)2(NO3)2(H2O)2]+. | ||
At the upper rim of C4AS, one 4,4′-bipyridine-N,N′-dioxide ligand is ‘perched’, the two pyridine rings twisted at an angle of 21.23°, since the cavity is too small to support lateral encapsulation of 4,4′-bpdo (Fig. 3a) which is transversely orientated with the N–O groups directed towards two opposing sulfonate groups of the C4AS. Both groups bridge the Sm2 and Sm3 cations.
There are non-classical hydrogen bonding interactions between the 4,4′-bpdo and the p-sulfonatocalix[4]arene, with the closest C–H⋯aromatic centroid distance of 3.290 Å in 1 (3.302 Å in 2). The p-sulfonatocalix[4]arene anion is present in its usual flattened cone conformation with C2v symmetry (cone angles between the planes of opposite aromatic rings are 65.18° and 82.28°). Accordingly, the largest angles between the planes of aromatic rings and the plane of the phenolic oxygen atoms are 133.53° in 1 (133.78° in 2).
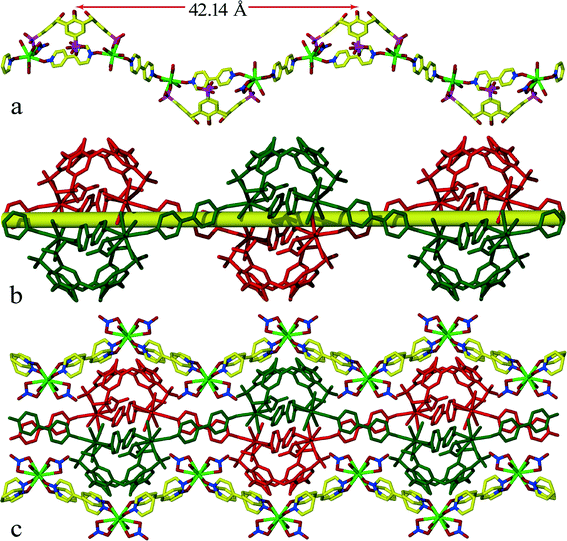
At pH ~5 p-sulfonatocalix[4]arene takes on an overall charge of −5, with two of the opposing sulfonate groups bonded to two Sm(III) cations. There are four nitrate anions which results in a total negative charge of −9 to balance with the three Sm(III) cations. It was not possible to locate all hydrogen atoms for disordered water molecules from the difference Fourier map as there was not enough electron density. Two Sm(III) ions connected by two bridging 4,4′-bpdo in a trans fashion and sulfonate group from C4AS form 1D chains which pack together in the crystal lattice to form infinite double stranded regular zigzag chains running along the c-axis (Fig. 4b). Each zigzag chain has a repeating motif of length of 42.14 Å (Fig. 4a). The interplay between chains is governed by 4,4′-bpdo⋯4,4′-bpdo transversely π-stacking interactions via lattice 4,4′-bpdo (centroid⋯centroid distance 3.95–4.25 Å), such that a 2D network of chains propagating along ac-plane is obtained throughout the crystal lattice this sheet structure is further sandwiched between the 2D sheets comprised of [Sm(4,4′-bpdo)2(NO3)2(H2O)2]+.
In the [Sm(4,4′-bpdo)2(NO3)2(H2O)2]+ 2D-sheets, four Sm(III) ions connected by four bridging 4,4′-bpdo ligands define the main skeleton of the 2D sheets (Fig. 4c and Fig. S1†) stacked together parallel to ac-plane. Adjacent sheets are linked together via the 1D chain through intermolecular hydrogen bonds and π⋯π-stacking interactions. The Sm–O distances for 4,4′-bpdo (2.25–2.39 Å) are shorter than those for C4AS (2.46–2.52 Å). The usual “up–down” relative arrangement of neighbouring p-sulfonatocalix[4]arene anions which is exhibited in the vast majority of structures containing this anion, is retained since the 4,4′-bdpo ligands of the 2D network simply act as spacers.
The lattice 4,4′-bpdo interacts with the 4,4′-bpdo ligated to Sm2 and Sm3 via π⋯π interaction (3.874–4.218 Å) to form two-dimensional sheets by joining the 1D chains of C4AS through the plane formed by these 4,4′-bpdo ligands as depicted in Fig. S2.†
Within the zigzag chain O25 from the sulfonate group in bifurcated mode interacts with H41a and H43a of the water molecules bonded to Sm3 via strong hydrogen bonds forming a six membered synthon of R12 (6) type (Fig. 5). Two strands also interact via strong hydrogen bond O14–H14a⋯O29 (dH⋯A = 1.85 Å, dD⋯A = 2.706(4) Å, ∠D–H⋯A = 167.8°, −x + 2, –y + 1, –z + 1) forming a slantwise capsular structure where both up–up facing C4AS from opposite strands are completely offset to each other. H16a of the water molecule bonded to Sm2 interact with O39 (dH⋯A = 2.03 Å) via a strong hydrogen bond (Tables S3 & S4†). ’The double strand zigzag chain interacts with the neighbouring parallel chain via these hydrogen bonds extending the network in the [100] direction. There is no direct hydrogen bonding interaction between the 1D chain network and 2D network within the lattice. However they interact via lattice water mediated hydrogen bonding. They also interact via π⋯π (centroid⋯centroid 3.969–4.290 Å) interaction between the C4AS aryl rings and the pyridyl rings of the 4,4′-bpdo ligand of 2D network. The O46 atom of the lattice 4,4′-bpdo is hydrogen bonded (dH⋯A = 1.93 Å, dD⋯A = 2.669(4) Å, ∠D–H⋯A = 146.9°, −x + 1, −y + 1, −z + 1) to the water hydrogen atoms of Sm3 forming an eight membered R23 (8) synthon mediated by lattice water O56. Interestingly, the extended structure of complex 1 and 2 shows infinitely extended hydrated channels running through [001] direction (Fig. S3†).
 | ||
| Fig. 5 Display of H-bonding between the 1D chains. H atoms (except for those involving hydrogen bonding) have been omitted for clarity. | ||
Powder X-ray diffraction (PXRD) patterns are provided in the ESI† for 1 and 2 (Fig. S6†). In both cases, the experimental patterns agree well with the calculated patterns. This indicates that the structure of the bulk material matches the single crystal X-ray structure for both 1 and 2. The experimental PXRD pattern of 2 does exhibit an ‘extra’ peak at ~6.5° 2θ, which may indicate the presence of a small impurity (e.g. a small amount of unreacted material), however, the rest of the pattern agrees well with the calculated PXRD pattern.
Topological studies
Sm(3) centre of [Sm(4,4′-bpdo)2(NO3)2(H2O)2]+ serves as a 4-connected node by linking to four 4,4-bipyridine-N,N-dioxide (4,4′-bpdo) ligands with the vertex symbol of [4.4.4.4.6(2).6(2)] to generate a 2-D sheet parallel to the ac-plane. Thus, a 2D (4)-connected network with the Schally symbol of {4^4·6^2}–VS[4·4·4·4·*·*] is generated with 4-c uninodal42sql/Shubnikov topology as depicted in Fig. 6.Thermal analysis
The TG data of complex 1 indicates two mass losses (Fig. S4†). The first mass loss 50–140 °C (8.464%) corresponds to the loss of all the lattice water molecules (calculated: 8.42%). A long range mass loss in the range 150–500 °C (20%) corresponds to the loss of all the coordinated water molecules along with the nitrate ion, subsequently followed by structural decomposition. DSC shows one broad endotherm peak which should be due to the loss of solvent molecules (Fig. S4†) and one broad exotherm due to structural decomposition. Hot stage microscopy indicates visual loss of water occurs for 1 and 2 at various temperatures (Fig. 7). The presence of lattice water molecules was also confirmed by the presence of broad peak at ~3400–3600 cm−1 IR spectrum (Fig. S5†).Conclusion
In summary, this work describes two isostructural mixed 1D–2D coordination polymers. In each case the structures are comprised of two components – a two-dimensional 4-connected framework made up of Sm/Nd nitrate centres bridged by 4,4′-bipyridine-N,N′-dioxide ligands and a one-dimensional chains of Sm/Nd nitrate centres bridged by 4,4′-bipyridine-N,N′-dioxide ligands and p-sulfonatocalix[4]arene ligands. Both supramolecular assemblies have been carefully studied from viewpoints of both complexation mode and extended structure. Interestingly, the well-known host molecule p-sulfonatocalix[4]arene, as part of the 1D polymer, acts as a guest for the 2D framework similar to the case of the Zhang et al. structure. Our present work provides 2 isostructural examples which show that this rare class of compounds can be extended. Moreover, it represents an advance in the complexity of these structures by way of the 1D polymerization of the p-sulfonatocalix[4]arene component with the retention of the hydrated channels. This may ultimately affect its porous properties and thus the present study contributes to the investigation of suitable manipulation factors in the pursuit of constructing functional solid-state materials in supramolecular chemistry and crystal engineering.Acknowledgements
A.H. and C.L.O. thanks the South African National Research Foundation and the University of Cape Town for financial support.References
- X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971–1981 CrossRef CAS PubMed.
- O. Danylyuk and K. Suwinska, Chem. Commun., 2009, 5799–5813 RSC.
- K. D. Daze and F. Hof, Acc. Chem. Res., 2013, 46, 937–945 CrossRef CAS PubMed.
- V. Francisco, N. Basílio and L. García-Río, J. Phys. Chem. B, 2014, 118, 4710–4716 CrossRef CAS PubMed.
- M. E. Belowich, C. Valente, R. A. Smaldone, D. C. Friedman, J. Thiel, L. Cronin and J. F. Stoddart, J. Am. Chem. Soc., 2012, 134, 5243–5261 CrossRef CAS PubMed.
- O. Danylyuk, H. Butkiewicz, A. W. Coleman and K. Suwinska, CrystEngComm, 2015, 17, 1745–1749 RSC.
- S. J. Dalgarno, M. J. Hardie, M. Makha and C. L. Raston, Chem. – Eur. J., 2003, 9, 2834–2839 CrossRef CAS PubMed.
- Z. Qin, D.-S. Guo, X.-N. Gao and Y. Liu, Soft Matter, 2014, 10, 2253–2263 RSC.
- Y. Liu, Z. Huang, X. Tan, Z. Wang and X. Zhang, Chem. Commun., 2013, 49, 5766–5768 RSC.
- T. Schrader, Nat. Chem., 2012, 4, 519–520 CrossRef CAS PubMed.
- P. Muthu Mareeswaran, E. Babu, V. Sathish, B. Kim, S. I. Woo and S. Rajagopal, New J. Chem., 2014, 38, 1336–1345 RSC.
- S. M. Taylor, R. D. McIntosh, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2012, 48, 11190–11192 RSC.
- R. Sun, C. Xue, M. Gao, H. Tian and Q. Li, J. Am. Chem. Soc., 2013, 135, 5990–5993 CrossRef CAS PubMed.
- J. Li, S. Zhang, Y.-G. Chen, X. Du, H. Yu and J. Yu, Inorg. Chem. Commun., 2014, 47, 93–95 CrossRef CAS PubMed.
- M. Liu, W. Liao, C. Hu, S. Du and H. Zhang, Angew. Chem., Int. Ed., 2012, 51, 1585–1588 CrossRef CAS PubMed.
- R. Mclellan, M. A. Palacios, C. M. Beavers, S. J. Teat, S. Piligkos, E. K. Brechin and S. J. Dalgarno, Chem. – Eur. J., 2015, 21, 1–10 CrossRef PubMed.
- N. Mattoussi, G. Pilet, G. Novitchi, F. Meganem and D. Luneau, Eur. J. Inorg. Chem., 2013, 2652–2656 CrossRef CAS.
- F. Gándara, E. Gutiérrez-Puebla, M. Iglesias, N. Snejko and M. A. Monge, Cryst. Growth Des., 2010, 10, 128–134 Search PubMed.
- Y. Bi, W. Liao, G. Xu, R. Deng, M. Wang, Z. Wu, S. Gao and H. Zhang, Inorg. Chem., 2010, 49, 7735–7740 CrossRef CAS PubMed.
- Y. Bi, X. T. Wang, W. Liao, X. Wang, R. Deng, H. Zhang and S. Gao, Inorg. Chem., 2009, 48, 11743–11747 CrossRef CAS PubMed.
- F. Perret and A. W. Coleman, Chem. Commun., 2011, 47, 7303–7319 RSC.
- J. L. Atwood, L. J. Barbour, M. J. Hardie and C. L. Raston, Coord. Chem. Rev., 2001, 222, 3–32 CrossRef CAS.
- D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907–5921 RSC.
- K. Wang and Y.-W. Yang, Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2013, 109, 67–87 RSC.
- R. E. McGovern, H. Fernandes, A. R. Khan, N. P. Power and P. B. Crowley, Nat. Chem., 2012, 4, 527–533 CrossRef CAS PubMed.
- R. E. McGovern, A. A. McCarthy and P. B. Crowley, Chem. Commun., 2014, 50, 10412–10415 RSC.
- K.-P. Wang, D.-S. Guo, H.-X. Zhao and Y. Liu, Chem. – Eur. J., 2014, 20, 4023–4031 CrossRef CAS PubMed.
- I. Ling, B. W. Skelton, A. N. Sobolev, Y. Alias and C. L. Raston, CrystEngComm, 2014, 16, 5159–5164 RSC.
- I. Ling, Y. Alias and C. L. Raston, New J. Chem., 2010, 34, 1802–1811 RSC.
- B. Jiang, D. Guo, Y. Liu, K. Wang and Y. Liu, ACS Nano, 2014, 8, 1609–1618 CrossRef CAS PubMed.
- G.-L. Zheng, G.-C. Yang, S.-Y. Song, X.-Z. Song and H. Zhang, CrystEngComm, 2014, 16, 64–68 RSC.
- G. Zheng, F. Zhang, Y. Li and H. Zhang, CrystEngComm, 2008, 10, 1560–1564 RSC.
- A. Husain and C. L. Oliver, CrystEngComm, 2014, 16, 3749–3757 RSC.
- Bruker (2007), Bruker AXS Inc., Madison, Wisconsin USA, 2007 Search PubMed.
- G. M. Sheldrick, TWINABS, Bruker AXS Inc., Madison, Wisconsin USA, 2001 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- A. V. Pavlishchuk, S. V. Kolotilov, I. O. Fritsky, M. Zeller, A. W. Addison and A. D. Hunter, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2011, 67, m255–m265 CAS.
- G. J. Palenik, Inorg. Chem., 2003, 42, 2725–2728 CrossRef CAS PubMed.
- K. Xiong, M. Wu, Q. Zhang, W. Wei, M. Yang, F. Jiang and M. Hong, Chem. Commun., 2009, 1840–1842 RSC.
- S. J. Dalgarno, J. L. Atwood and C. L. Raston, Cryst. Growth Des., 2006, 6, 174–180 CAS.
- G. Zheng, H.-J. Zhang, S.-Y. Song, Y.-Y. Li and H.-D. Guo, Eur. J. Inorg. Chem., 2008, 1756–1759 CrossRef CAS.
- L. Qin, J. Zheng, S.-L. Xiao, X.-H. Zheng and G.-H. Cui, Inorg. Chem. Commun., 2013, 34, 71–74 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information available: Crystal and geometrical data, structural depictions, TGA and DSC curves, PXRD patterns and an IR spectrum for 1. CCDC reference numbers 1035921 and 1035922 for 1 and 2, respectively. See DOI: 10.1039/c5ce00153f |
| This journal is © The Royal Society of Chemistry 2015 |