Complex three-dimensional lanthanide metal–organic frameworks with variable coordination spheres based on pyrazine-2,3,5,6-tetracarboxylate†
Abstract
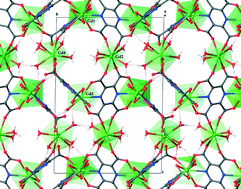
Metal–organic frameworks {[Ln4(pztc)3(H2O)11]·10(H2O)}n (Ln = Gd(1), Tb(2); pztc = pyrazine-2,3,5,6-tetracarboxylate) containing variable coordination spheres and with a complex and unusual three dimensional structure, were synthesized by the reaction of H4pztc with the respective Ln(III) salt in water under hydrothermal conditions. The compounds were characterized by single crystal X-ray crystallography, elemental and thermal analysis, and FTIR spectroscopy. The asymmetric units in these compounds have four symmetry-independent Ln(III) ions and these are octa- and nona-coordinate centers, with irregular coordination polyhedra from [Ln(pztc)2(H2O)6], [Ln(pztc)2(H2O)4], [Ln(pztc)3(H2O)3], [Ln(pztc)3(H2O)], and [Ln(pztc)4] cluster units. The fully deprotonated ligand, pztc, coordinates to the Ln3+ ions through seven or through ten of its atoms (i.e., the maximum coordination number for this ligand). The three-dimensional open framework contains irregular channels along the [001] crystallographic direction. The channels are approximately 12 Å wide at their largest dimension and contain strongly hydrogen bonded water molecules of crystallization which further stabilize the structure. The solvent accessible volume is 20% of the total volume. The structures exhibit magnetic behavior that is characteristic of the respective isolated paramagnetic lanthanide ions.

 Please wait while we load your content...
Please wait while we load your content...