Self-triggered conformations of disulfide ensembles in coordination polymers with multiple metal clusters†
Abstract
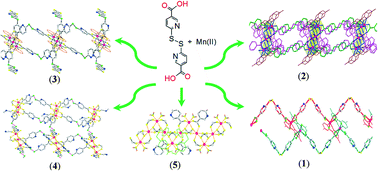
Five coordination polymers, {[Mn2(dtdn)2(4,4′-dmbpy)2(H2O)2]·DMF}n (1, dtdn = 6,6′-dithiodinicotinate), {[Mn6(dtdn)6(5,5′-dmbpy)4]·5DMF·2H2O}n (2), {[Mn3(dtdn)2(Hdtdn)2(phen)2]·H2O}n (3), {[Mn6(dtdn)6(H2O)4(DMF)4]·10DMF·10H2O}n (4), and {[Mn2(dtdn)2(H2O)2]·4MeOH·H2O}n (5) (4,4′-dmbpy = 4,4′-dimethyl-2,2′-bipyridine, 5,5′-dmbpy = 5,5′-dimethyl-2,2′-bipyridine, phen = 1,10-phenanthroline), were constructed under mild reaction conditions from Mn(II) ions and a twisted disulfide ligand, H2dtdn, in the presence of different ancillary N-donor coligands. These compounds show a variety of guest inclusion properties and interesting self-induced chirality. Compound 1 adopts 1D zigzag chains with mononuclear units that are mutually paired into a 1D ladder-like structure. Compound 2 consists of trinuclear clusters, and displays a 2D protuberant sheet with a (4,4)-sql topology. Compound 3 takes the form of a 1D double-stranded chain with rectangular loops comprised of trinuclear clusters, in which the Hdtdn− ligands dangle on both sides. Compound 4 adopts the form of a 2D network and exhibits a slightly undulating (44) topology that consists of trinuclear clusters, which are further hydrogen bonded into a 3D framework. Compound 5 features a 3D network consisting of 1D infinite metal oxide wires containing the [Mn2(dtdn)2(H2O)2] unit. Magnetic studies of compounds 2, 3, and 5 show that they have antiferromagnetic properties.

 Please wait while we load your content...
Please wait while we load your content...