Open Access Article
Open Access ArticleConformational adaptations of acyclic receptor templated by weakly coordinating anions†
I.
Đilović
*a and
K.
Užarević‡
*b
aDepartment of Chemistry, Faculty of Science, University of Zagreb, Horvatovac 102a, 10000 Zagreb, Croatia. E-mail: idilovic@chem.pmf.hr
bRuđer Bošković Institute, Division of Physical Chemistry, Bijenička 54, 10002 Zagreb, Croatia. E-mail: Krunoslav.Uzarevic@irb.hr
First published on 16th March 2015
Abstract
Crystallization from methanol solutions resulted in new supramolecular complexes of a flexible polyamine receptor N,N′-3-azapentane-1,5-bis[3-(1-aminoethylidene)-6-methyl-3H-pyran-2,4-dione] (L) with spherical (Br− and I−), linear (SCN−) or pyramidal (ClO3−) anions. The recognition and self-assembly processes yielded five solids: solvated (1a) and non-solvated (1b) forms of HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCN− complex with quite different receptor conformations, a supramolecular complex with ClO3− anion (3), which is isoskeletal to 1b, and HL
SCN− complex with quite different receptor conformations, a supramolecular complex with ClO3− anion (3), which is isoskeletal to 1b, and HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br− (2) and HL
Br− (2) and HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I− complexes which are isoskeletal to 1a. It is shown that the rich coordinating potential of the anions investigated arises not only from their geometry but also from their ambivalent accepting functionalities. The divergence in the way binding occurs is reflected on supramolecular synthons stabilizing crystal structures as well as on the physicochemical properties of the obtained solids. In these systems, anion binding could be interpreted as a competition between two “solvation spheres”, one provided by the solvent molecules and the other dominated by high-order supramolecular receptors built from HL molecules. This behaviour is related to previously reported complexes of HL with trigonal, tetrahedral and octahedral anions, emphasizing the receptor's conformational adaptability for providing the most favourable surrounding for guest inclusion in the rigid environment of a crystal.
I− complexes which are isoskeletal to 1a. It is shown that the rich coordinating potential of the anions investigated arises not only from their geometry but also from their ambivalent accepting functionalities. The divergence in the way binding occurs is reflected on supramolecular synthons stabilizing crystal structures as well as on the physicochemical properties of the obtained solids. In these systems, anion binding could be interpreted as a competition between two “solvation spheres”, one provided by the solvent molecules and the other dominated by high-order supramolecular receptors built from HL molecules. This behaviour is related to previously reported complexes of HL with trigonal, tetrahedral and octahedral anions, emphasizing the receptor's conformational adaptability for providing the most favourable surrounding for guest inclusion in the rigid environment of a crystal.
Introduction
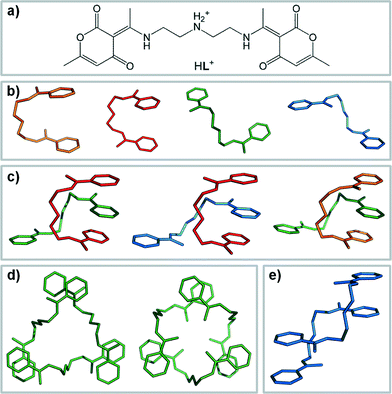
The design of artificial receptors which are able to recognize, bind, sense and/or transport guest molecules is attracting increasing attention.1 A great variety of preorganized and rigid anion receptors has been studied, as these systems often display high peak selectivity in solution.2 The second class of often studied receptors comprises those with the ability to change their conformation in order to respond to the specific demands of the anionic template (geometry, charge density, pH-dependent behaviour, etc.).3 These receptors often have lower binding constants in contrast to their rigid analogues, but they showed strong potential in the development of various functional supramolecular systems,4 from sensors and signallers5 to more effective rigid receptors.6 In this context, the study of the interaction modes between the flexible receptor and flexible or multifaceted anions (such as linear thiocyanate or pyramidal chlorate) could prove especially interesting and lead to further developments in the field.7–10In our recent studies of binding of environmentally relevant anions, flexible polyamine receptor HL (Scheme 1a) was found to display a remarkable adaptability towards diverse inorganic or organic anions, with high peak selectivity for hydrogen maleate, sulphate and nitrate.11–13 Two principal sets of the receptor conformations were observed in the obtained crystal structures, denoted as C- and Z-conformers (Scheme 1b). Occasionally, a single HL molecule served as a receptor for a single anion (hydrogen maleate or o-phthalate), but more often, HL cations assembled to supramolecular receptors, in order to achieve complementarity for specific anion(s). The observed supramolecules include discrete CZ-dimers, various Z-trimers and supramolecular polymers (Scheme 1c–e). Hydrophilic anions (trigonal NO3− and tetrahedral SO42−) were excluded from the competitive mixtures by the self-assembly of three HL molecules into a pseudo-macrocycle, with the anion encapsulated inside the formed receptor supramolecule by 12 strong N–H⋯O hydrogen bond interactions (Scheme 1d, left).11 On the other hand, perchlorate anion (ClO4−), which is geometrically identical to sulphate but with a lower charge density, templates the self-assembly of three HL cations into a new supramolecular receptor with vacant interior, and the binding is achieved by weak C–H donor groups (Scheme 1d, right).12a The same binding mode was found for other hydrophobic anions (HSO3−, IO4−, PF6−, SbF6−) which were encapsulated in similar hydrophobic pockets of the emerged crystal structure.12a In the systems studied,11,12 HL proved highly sensitive to the geometry and charge density of the anionic guest. Selectivity in separation has usually been achieved only upon crystallization, emphasizing the importance of intermolecular interactions in the rigid crystal environment which lock the conformation of the host. The same supramolecular concept was also transferred to solvent-free mechanochemical discrimination between isomeric organic acids.13
Herein we study the complexation modes of the receptor HL with spherical (Br− and I−) and multifaceted linear (SCN−) or pyramidal (ClO3−) anions. The structural investigations on two forms of HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCN− complexes (1a and 1b) have shown that neither anion geometry nor the overall charge is decisive for the final folding mode of the receptor. The results indicate that the types of accepting groups on the anion also play an important role in the occurring type of complex. The interplay between anion coordination ambivalence and receptor conformational/binding versatility results in different host–guest interactions and supramolecular architectures, where the receptor molecules assemble to polymeric chains (as in 1a and 2 – with SCN−, Br− and I−) or dimers (1b and 3 – with SCN− and ClO3−). To rationalize our structural observations, detailed overview of the Cambridge Structural Database (CSD)14 and Protein Data Bank (PDB)15 was made, and the results were compared with already known host/protein and SCN− supramolecular complexes.
SCN− complexes (1a and 1b) have shown that neither anion geometry nor the overall charge is decisive for the final folding mode of the receptor. The results indicate that the types of accepting groups on the anion also play an important role in the occurring type of complex. The interplay between anion coordination ambivalence and receptor conformational/binding versatility results in different host–guest interactions and supramolecular architectures, where the receptor molecules assemble to polymeric chains (as in 1a and 2 – with SCN−, Br− and I−) or dimers (1b and 3 – with SCN− and ClO3−). To rationalize our structural observations, detailed overview of the Cambridge Structural Database (CSD)14 and Protein Data Bank (PDB)15 was made, and the results were compared with already known host/protein and SCN− supramolecular complexes.
Results and discussion
Crystalline products 1a and 1b were obtained from the self-assembly between L and ammonium thiocyanate in acidified methanol (both 1a and 1b) or ethanol (1b exclusively) solutions. From methanol, the occurrence of the respective phase was related to the time needed for crystallization. The slow evaporation and prolonged duration of crystallization resulted in exclusive occurrence of the solvated phase, 1a, whereas the fast crystallization from highly concentrated methanol solutions yielded 1b. From ethanol, crystallization would proceed swiftly (in a matter of minutes) and 1b was harvested in all cases. Reactions were also attempted in acetonitrile and acetone, where the ligand, NH4SCN and p-toluenesulfonic acid are readily soluble. However, from these solvents solid ammonium p-toluenesulfonate was obtained. Complexes with bromide and chlorate anions, 2 and 3 respectively, were obtained by reacting equimolar amounts of L and HBr or HClO3 in methanol. Colourless crystals of forms 1a, 2 and 3 desolvated and fell apart in air, while 1b was stable, even at 80 °C. The complex of HL with iodide anions was prepared by a procedure similar to 1a, but the obtained crystals were not suitable to conduct a thorough structural analysis and will therefore be excluded from detailed discussion. However, the derived structural model was sufficient to establish the unit cell parameters and the arrangement of the receptor molecules, which is very similar to that of the bromide complex 2 and the 1a form of the SCN− complex. The IR spectra of bromide and iodide complexes are in a good agreement, further suggesting that the same supramolecular synthon is present in the solid phase (for more details, please refer to the ESI†).Crystal structure description
In four crystal structures, three distinguishable HL conformers were found (Tables 1 and 2). In 1b and 3, syn-C and syn-Z conformers assemble to CZ-dimers which are favorable for anion binding [Scheme 1c (far right) and Fig. 2]. In 1a and 2, on the other hand, syn-Z conformers occur exclusively, building [syn-Z⋯syn-Z]n supramolecular polymers through the rich pattern of noncovalent interactions. The key structural parameters (bond lengths, hydrogen-bonding geometry) are given in Tables S1–S8 in the ESI.†| syn-Z (1a) | syn-Z (1b) | syn-C (1b) | syn-Z (2) | syn-Z (3) | syn-C (3) | |
|---|---|---|---|---|---|---|
a
φ
1 ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N11–C19–C110–N12; φ2 N11–C19–C110–N12; φ2 ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C19–C110–N12–C111; φ3 C19–C110–N12–C111; φ3 ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C110–N12–C111–C112; φ4 C110–N12–C111–C112; φ4 ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C12–C111–C112–N13. C12–C111–C112–N13.
|
||||||
| φ 1 | 56.9(4) | 65.6(3) | 72.9(3) | 56.4(7) | 53.6(4) | 74.9(3) |
| φ 2 | 174.2(3) | 87.2(3) | 171.5(2) | 174.5(5) | 161.3(3) | 173.0(3) |
| φ 3 | 173.5(3) | 171.7(2) | 171.7(2) | 172.9(5) | 177.9(3) | 176.6(3) |
| φ 3 | 54.2(4) | 51.5(3) | 71.7(3) | 53.4(7) | 55.9(4) | 71.6(3) |
| 1a | 1b | 2 | 3 | |
|---|---|---|---|---|
| a R = ∑||Fo| − |Fc||/∑Fo, w = 1/[σ2(Fo2) + (g1P)2 + g2P], where P = (Fo2 + 2Fc2)/3; S = ∑[w(Fo2 − Fc2)2/(Nobs − Nparam)]1/2. | ||||
| Formula | C22H30N4O7S | C21H26N4O6S | C20H26N3O6Br | C43H64Cl2N6O21 |
| Crystal size (mm3) | 0.15 × 0.12 × 0.10 | 0.21 × 0.18 × 0.15 | 0.30 × 0.10 × 0.03 | 0.25 × 0.22 × 0.13 |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
C2/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n |
| Temperature (K) | 120 | 293 | 150 | 293 |
| Unit cell dimensions | ||||
| a (Å) | 8.0072(5) | 33.554(7) | 7.9370(5) | 15.9465(6) |
| b (Å) | 11.8432(9) | 13.996(3) | 12.8236(10) | 18.9851(4) |
| c (Å) | 13.3307(9) | 18.645(4) | 13.4836(10) | 17.1610(7) |
| α (°) | 96.449(6) | 90.00 | 79.804(6) | 90.00 |
| β (°) | 106.07(6) | 100.54(3) | 76.806(6) | 111.55(1) |
| γ (°) | 97.869(6) | 90.00 | 78.841(6) | 90.00 |
| Volume (Å3) | 1188.2(1) | 8608(3) | 1298.1(1) | 4862.2(3) |
| Z | 2 | 16 | 2 | 4 |
| D calc (g cm−3) | 1.382 | 1.428 | 1.249 | 1.473 |
| μ (mm−1) | 0.187 | 0.198 | 1.669 | 0.223 |
| F(000) | 524 | 3904 | 478 | 2264 |
| Refl. collected/independent | 8861/4543 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162/8419 162/8419 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549/2847 549/2847 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110/8464 110/8464 |
| No. observed refl. [I > 2σ(I)]a | 2443 | 4099 | 1566 | 6653 |
| No. restraints/no. parameters | 0/309 | 0/595 | 0/275 | 6/668 |
| R/wR [I > 2σ(I)]a | 0.0732/0.1353 | 0.0481/0.0749 | 0.1041/0.3058 | 0.0665/0.1859 |
| R/wR [all data] | 0.1560/0.1589 | 0.0872/0.1291 | 0.1452/0.3386 | 0.0816/0.1971 |
| Goodness-of-fit on F2 | 0.970 | 0.841 | 1.161 | 1.033 |
| Largest diff. peak and hole (e Å−3) | 0.639, −0.387 | 0.297, −0.322 | 1.146, −0.902 | 1.192, −0.538 |
Supramolecular polymers for anion exclusion
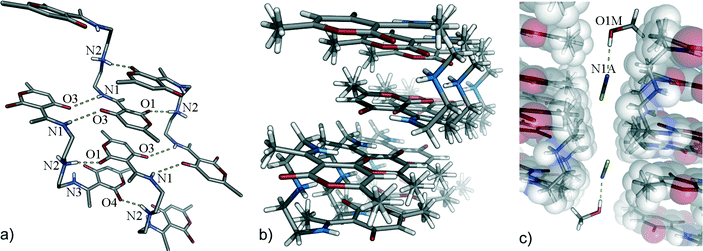
In complexes with SCN− (1a) and Br− (2), exclusively syn-Z conformers self-assemble to a new kind of polymeric host for a complexation of SCN− and Br− anions (Fig. 1). The central amino –NH2+ group acts as a HB-bridge between two distinct pyrone oxygen atoms [O1 and O4, 2.715(4) and 2.698(4) Å, respectively] in a one-dimensional chain. Neighbouring chains are further connected by the N1–H1⋯O3 [3.042(4) Å] and N2–H2⋯O6 [3.091(4) Å] hydrogen bonds [R22(12) type] and pyrone π⋯π interactions [3.454(2) and 3.265(2) Å]. This layer-like arrangement of HL molecules saturates the strong N–H donors and creates large C–H rich “walls”, parallel to the crystallographic ac plane, in which the anions are accommodated. These hydrophobic regions are also occupied by the solvent methanol molecules which anchor the SCN− by strong hydrogen bonds [O1M–H1M⋯N1A, 2.837(5) Å]. The other side of SCN− is coordinated using preferentially weaker C–H⋯S1A and C–H⋯C1A interactions from HL. A picture emerges in which anion binding could be interpreted as a competition between two “solvation spheres”, one provided by the solvent molecule and the other dominated by highly ordered supramolecular receptors built from HL cations. The bromide complex 2 is isostructural to 1a (for details, please see the experimental section).Anion encapsulation by CZ-dimers
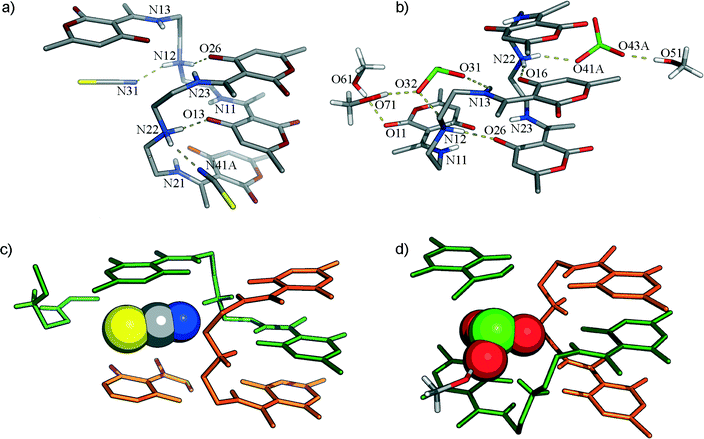
In both crystal structures 1b and 3, dimers are made of syn-C and syn-Z conformers constructing interpenetrating chains (mutually held by C–H⋯O and C–H⋯π interactions) which are capable of encapsulating anions in the emerged voids (Fig. 2). In a single chain of 1b, syn-C conformers act as π-molecular tweezers for syn-Z conformers, which are anchored by a strong charge-assisted hydrogen bond [N22–H22A⋯O13, 2.792(3) Å].It seems again13 that the formation of tweezers is advantageous for planar HB-acceptors. The central –NH2+ group is the best donor and with the best acceptor (pyrone O13-atom) forms a short intermolecular hydrogen bond, while the receptor supermolecule is completed through pyrone π⋯π interactions [3.56(1) and 3.55(1) Å]. In 1b, a second hydrogen atom of the –NH2+ group acts as a bifurcated hydrogen bond donor towards the nitrogen atom of SCN− [2.914(15) Å] and O11 [2.889(3) Å] of the other Z-conformer. In the Z-conformers, the central –NH2+ group forms hydrogen bonds with O26 [2.881(3) Å] atoms from C-conformers and nitrogen atoms (N31) of other SCN− anions [2.688(3) Å]. It is also important to address how the SC-part, particularly the S-atomic part, of the SCN− is accommodated in a highly complementary environment within the crystal, i.e. in C–H rich surfaces inside the crystal structure. The other sulphur atom of the crystallographically independent SCN−, in addition to the C–H region, is embraced by the π-systems of two neighbouring pyrone moieties [3.36(1) Å from the Z-conformer and 3.34(1) Å from the C-conformer, Fig. 4].
It is interesting to note that, when the main interaction of the receptor is with the weaker hydrogen-bonding acceptor group of the thiocyanate, the receptor self-assembles to form a high-ordered supramolecule (polymer) in order to saturate its strong hydrogen-bonding donor groups and uses only weaker C–H donors for anion binding. When the receptor binds the thiocyanate guest over the strong hydrogen-bonding acceptor, its conformation is adapted to accomplish the binding with strong N–H donor groups.
Conformational analysis
In a great extent, crystal structure 3 resembles 1b, except having three methanol molecules per CZ-dimer. Aliphatic chain torsion angle analysis for all conformers shows fine details in conformational adaptability of the receptor for the specific anion (Table 1 and Fig. 3). For both C-conformers in 1b and 3, torsion angle values fall within a very narrow angular window. The Z-conformers, roughly, could be divided into two pairs, depending on the distance between the centres of two pyrone rings. More compact ones are almost the same and are used as building blocks in 1a and 2 {new polymers of a [syn-Z⋯syn-Z]n type}. The others found in CZ-dimers show significant differences.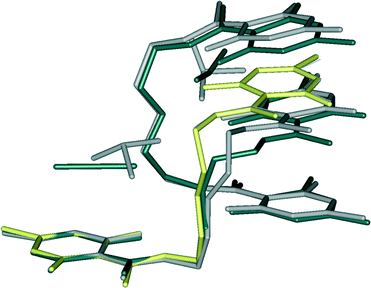 | ||
| Fig. 3 Overlay of the molecular structures from 1a (yellow), 1b (dark grey), 2 (light blue) and 3 (grey). Hydrogen atoms are not shown. | ||
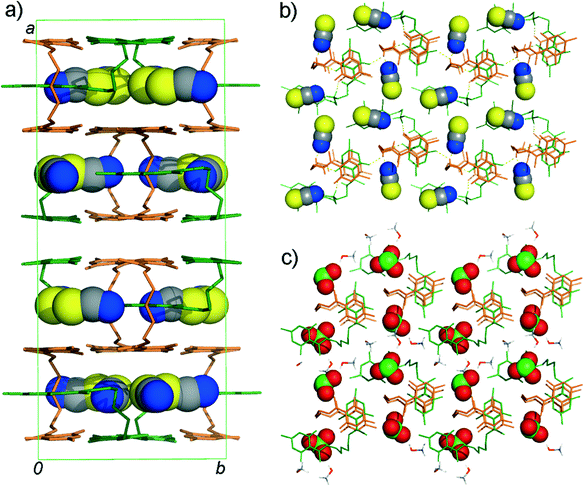
Thiocyanate is placed almost parallel to the line defined by the centres of pyrone and RAHB-ring giving rise to aromatic interactions. Although it is as close to the Z-conformer as SCN−, ClO3− simply needs more space which causes the slight shift in the upper part of the aliphatic chain (as in Fig. 3) so that “tweezed” pyrone moiety maintains the same position. To encapsulate a pyramidal ClO3− anion, the distance between two Z-conformers must be greater (in 3, it is longer for about 0.3 Å than in 1b). This opening results in an additional space which becomes populated by methanol molecules. Accommodated solvent molecules provide a source of HB-donors for the remaining oxygen atoms of the chlorate. Crystal packing of forms 1b and 3 are shown in Fig. 4.
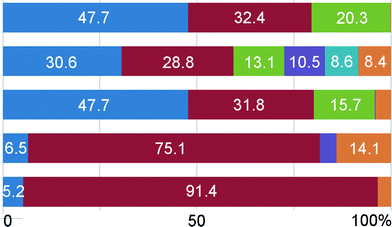
Hirshfeld surface analysis
Differences in the anion environment and the details of intermolecular hydrogen bonding in structures 1a, 1b and 3 can be extracted from decomposed two-dimensional fingerprint plots.16Scheme 2 shows the contribution (in %) to the Hirshfeld surface area for a distinct type of intermolecular contact for SCN− and ClO3− anions in the HB-networks 1a, 1b and 3. S⋯H contacts comprise 47.7% of the surface area in 1a and 1b (Z-conformers) and 30.6% in 1b (C-conformer). For C-conformer, the contribution of N⋯H contacts to the total surface area is smaller. This is largely compensated with the increase in the surface area involved in the π⋯π interactions. In 3, anions are mostly connected to the hydrophilic amino and hydroxyl groups. One ClO3− anion is additionally placed between two π-systems from HL+ molecules giving rise to the anion⋯π interactions.IR spectroscopy
In the spectra of all complexes, the sharp N–H band at 3123 cm−1 disappears upon complexation, indicating that the central amino group now participates in hydrogen bonding. For 1a, where the SCN− nitrogen atom is an acceptor of the strong hydrogen bond from the methanol hydroxyl group [O(methanol)⋯N(SCN−) distance is 2.837 Å], the band corresponding to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibration is a sharp band found at 2054 cm−1. For 1b, where two crystallographically independent SCN− ions are hydrogen bonded by protonated amino groups of the C- and Z-conformers of the host [with N(amino)⋯N(SCN−) distances of 2.698 Å and 2.926 Å, respectively], this band can be found at 2053 cm−1 with a wide shoulder around 2037 cm−1. However, regardless of the anion present in the structure, the band pattern in the range of 1700–500 cm−1 depends largely on the specific type of host conformation or supramolecular self-assembly.
N stretching vibration is a sharp band found at 2054 cm−1. For 1b, where two crystallographically independent SCN− ions are hydrogen bonded by protonated amino groups of the C- and Z-conformers of the host [with N(amino)⋯N(SCN−) distances of 2.698 Å and 2.926 Å, respectively], this band can be found at 2053 cm−1 with a wide shoulder around 2037 cm−1. However, regardless of the anion present in the structure, the band pattern in the range of 1700–500 cm−1 depends largely on the specific type of host conformation or supramolecular self-assembly.
1a and 2 (as well as in the HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I− complex) have almost identical arrangement of the host cations, where HL is exclusively in syn-Z conformation and builds chains along the crystallographic a-axis. The lactone oxo-group in these chains is involved in the strong hydrogen bond from the central protonated amino group of the other HL, resulting in the shift of the corresponding band from 1690 cm−1 in L to 1683 cm−1 in these complexes. In 1b, where the HL assembles to syn-C–syn-Z dimer, the same band can be found at 1700 cm−1. Other bands, corresponding to C
I− complex) have almost identical arrangement of the host cations, where HL is exclusively in syn-Z conformation and builds chains along the crystallographic a-axis. The lactone oxo-group in these chains is involved in the strong hydrogen bond from the central protonated amino group of the other HL, resulting in the shift of the corresponding band from 1690 cm−1 in L to 1683 cm−1 in these complexes. In 1b, where the HL assembles to syn-C–syn-Z dimer, the same band can be found at 1700 cm−1. Other bands, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations, are found at 1654, 1602, 1571 (broad doublet) and 1480 cm−1 in 1a and 1b. In 1b, these bands are shifted to 1653, 1612, 1569 (sharp singlet) and 1474 cm−1. The band pattern in 1b was compared to the previously observed complex of HL with hydrogen fumarate, where the HL was assembled in an almost identical syn-C–syn-Z dimer.13 The investigated bands in this complex are identical to those from 1b, with slight differences assigned to bonded fumarate (Fig. S1 and S2†).
N vibrations, are found at 1654, 1602, 1571 (broad doublet) and 1480 cm−1 in 1a and 1b. In 1b, these bands are shifted to 1653, 1612, 1569 (sharp singlet) and 1474 cm−1. The band pattern in 1b was compared to the previously observed complex of HL with hydrogen fumarate, where the HL was assembled in an almost identical syn-C–syn-Z dimer.13 The investigated bands in this complex are identical to those from 1b, with slight differences assigned to bonded fumarate (Fig. S1 and S2†).
For the ClO3− complex 3, the lactone oxo-group band can be observed at 1699 cm−1, and the IR pattern for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations resembles that from 1b. All four characteristic vibration modes for the ClO3− anion are clearly visible in the spectrum, the Cl–O symmetric stretch at 939 cm−1 as a moderately strong band, Cl–O asymmetric stretch at 980 cm−1 as a broad and strong band, and the “umbrella” vibration at 607 cm−1. The broadening of the signals can be tentatively assigned to the presence of two differently bonded ClO3− anions in the structure and the overlapping of their vibration bands. The inspection of the infrared spectra for the obtained forms revealed that for these systems this technique could be extremely informative not only for establishing the composition of the supramolecular complex and primary interactions between host and guest on the molecular level19 but also for establishing the mode of the folding and self-organization between the HL cations. Each of the observed conformers or supramolecular assembly types of HL has a unique spectrum. Since the majority of these compounds are air-sensitive solvates, PXRD data are often ambiguous. IR spectra can thus provide a quick insight into the arrangement of the host molecules.
N vibrations resembles that from 1b. All four characteristic vibration modes for the ClO3− anion are clearly visible in the spectrum, the Cl–O symmetric stretch at 939 cm−1 as a moderately strong band, Cl–O asymmetric stretch at 980 cm−1 as a broad and strong band, and the “umbrella” vibration at 607 cm−1. The broadening of the signals can be tentatively assigned to the presence of two differently bonded ClO3− anions in the structure and the overlapping of their vibration bands. The inspection of the infrared spectra for the obtained forms revealed that for these systems this technique could be extremely informative not only for establishing the composition of the supramolecular complex and primary interactions between host and guest on the molecular level19 but also for establishing the mode of the folding and self-organization between the HL cations. Each of the observed conformers or supramolecular assembly types of HL has a unique spectrum. Since the majority of these compounds are air-sensitive solvates, PXRD data are often ambiguous. IR spectra can thus provide a quick insight into the arrangement of the host molecules.
Thermal behaviour
The solvated form of the thiocyanate complex, 1a, was subjected to DSC and TGA measurements prior to the crystalline methanol loss. The laboratory was cooled to 10 °C and the crystals in their mother liquid were kept at 4 °C. Crystals of 1a were taken from their mother liquid and immediately dried in methanol vapours, in order to remove methanol from their surface and to prevent egress of methanol from the crystal structure. The prepared sample was placed in 40 μL aluminium crucibles and sealed with a perforated aluminium cover. The DSC curve revealed two thermal events just before 130 °C which are followed by a stronger endotherm at 190 °C (melting). We collected a sample at 140 °C and analysed it by PXRD and IR. No significant changes were observed in IR spectra, and the PXRD diffractogram showed a partial amorphization of the compound. However, no thermally induced transformation of 1a to 1b was observed. Compound 2 showed almost identical thermal behaviour to 1a. Compound 1b was stable below 195 °C (onset), where the strong endotherm was ascribed to the melting. For complex 3, no thermal measurements were performed due to its potential explosive properties.Conclusions
We have demonstrated how geometrically simple anions such as SCN− and ClO3− can serve as versatile templates for building divergent hydrogen-bonding networks. The complex coordinating potential of these anions arises primarily from their ambivalent hydrogen-bond or π-accepting properties. Depending on the available anion accepting group (e.g. S- or N-atoms in SCN−), the flexible HL molecules respond by adopting different conformers or self-organization modes, to provide a rigid and complementary environment for the most efficient binding in the formed crystal architecture. In form 1a, SCN− uses a sulphur atom as an acceptor and all strong hydrogen bond donors of the HL become (coordinatively) saturated by interactions with the oxo-functionalities from other HL molecules, so only the weaker directional binding groups are accessible for anion binding. On the other hand, when SCN− uses a nitrogen atom as a hydrogen-bonding acceptor (form 1b), then HL changes its geometry in order to employ strong donor groups for optimal binding of SCN−. In terms of number of potential hydrogen bond acceptors, chlorate anion is more demanding. Hydrogen bonding saturation is completed through interaction with solvent molecules in 3. Although it is hard to expect control over the local geometry in the solid state, the results show how multifaceted anions can be used as complex anionic templates for building new and diverse supramolecular systems.Experimental
Materials
All chemicals (Aldrich) used for the syntheses were at least of analytical grade and used as purchased. Solvents (Kemika) were distilled before use.General synthetic procedures
L was synthesized by the method described in the literature.20 The purity of the sample was checked by IR and UV-vis analyses. Complexes 1a, 1b and 2 were obtained by self-assembly processes in the methanol solutions. All syntheses were conducted at room temperature. Complex 1a was prepared by addition of NH4SCN (0.1 mmol) to the methanol solution of L (0.062 mmol in 4 mL of methanol) acidified by an equimolar amount of p-toluenesulfonic acid (yield, based on L = 67%). Colourless prisms readily lose the crystalline solvent after a short exposure to air. Complex 1b was prepared by an identical procedure with the only difference being that KSCN was used as a source of SCN− anions (yield = 82%). The same product was obtained by reaction of NH4SCN and L in acidified methanol (0.062 mmol in 2 mL, yield = 76%) or as a microcrystalline product from EtOH (yield = 90%). 1b crystallizes as colourless plates, which are stable upon exposure to air. Crystals of 1a and 1b, appropriate for diffraction studies, were obtained from mother liquids. Complex 2 was obtained by addition of an equimolar amount of HBr to the methanol solution of L (0.062 mmol in 2.4 mL of methanol). Colourless prisms were obtained after the solution was left to stand for 1 day at room temperature (87%). Crystals appropriate for diffraction studies were collected from diluted methanol solution (0.062 mmol of starting compounds in 8 mL of methanol). The iodide complex was obtained by reaction of equimolar amounts of L and tetrabutylammonium iodide in acidified methanol solution (0.062 mmol of p-toluenesulfonic acid in 3 mL of methanol). Complex 3 was obtained by the following procedure: Ba(ClO3)2 (0.040 mmol) was dissolved in 3 mL of methanol, and sulphuric acid (0.07 mmol) was added to the reaction mixture. The white precipitate (BaSO4) was filtered. Then, 0.062 mmol of L was dissolved in 3 mL of methanol and was added to the clear filtrate. The colourless crystals, which would decompose when dried, were obtained from their mother liquid after the solution was left to stand for 7 days at RT (yield = 37%). The crystals were suitable for diffraction studies.IR spectroscopy
IR spectra were recorded on a PerkinElmer Spectrum RXI FT-IR spectrometer (KCl pellet technique, 4000–400 cm−1 range, 2 cm−1 step). L: 3432 (broad) (νN–H, RAHB); 3366(sharp) (νN–H, central) 1688, 1656, 1636, 1574, 1472 (all νs, mixed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1a: 3466, 3353 (br) (νN–H, hydrogen bonded); 2052 (νC
C). 1a: 3466, 3353 (br) (νN–H, hydrogen bonded); 2052 (νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N); 1682, 1652, 1601, 1571 (all νs, mixed C–O, C
N); 1682, 1652, 1601, 1571 (all νs, mixed C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1b: 3440 (br) (νN–H, hydrogen bonded); 2053 (νC
C). 1b: 3440 (br) (νN–H, hydrogen bonded); 2053 (νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N); 1700, 1655, 1612, 1567 (all νs, mixed C–O, C
N); 1700, 1655, 1612, 1567 (all νs, mixed C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 2: 3440 (br) (νN–H, hydrogen bonded); 1676, 1640, 1576 (all νs, mixed C–O, C
C). 2: 3440 (br) (νN–H, hydrogen bonded); 1676, 1640, 1576 (all νs, mixed C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 843 (s) (νP–F).
C), 843 (s) (νP–F).
TGA and DSC experiments
Thermogravimetric analyses were performed using a Mettler-Toledo TGA/SDTA851e thermobalance with aluminium crucibles under nitrogen stream with a heating rate of 5 °C min−1. The results were processed using the Mettler STARe 9.01 software. DSC measurements were performed under a nitrogen stream using a Mettler-Toledo DSC823e calorimeter with STARe SW 9.01 (5 °C min−1).Single-crystal X-ray crystallography
Compounds 1a, 1b, 2 and 3 were obtained from methanol as colourless prismatic crystals at room temperature (Table 2). Each single crystal was mounted on a glass fibre and used for measurements of unit cell parameters and intensity data collection. Diffracted intensities were collected using an Oxford Diffraction Xcalibur 3 CCD diffractometer with graphite-monochromated MoKα (0.71073 Å) radiation at 120, 150 and 293 K. The CrysAlis21 program package was used for data collection, cell refinement and data reduction. Multi-scan empirical absorption corrections were applied using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm of CrysAlis. Structures were solved by direct methods (SHELXS22) and refined with full-matrix least-squares on F2 (SHELXL-97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and 2014
22 and 2014![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22b,c), using the WinGX23 program package. All non-hydrogen atoms were refined anisotropically. All hydrogen atoms were assigned calculated positions and allowed to ride on their carrier atoms. There are two versions of structure 2, one with modelled disordered molecules (presented only in the ESI†) and the other one in which the electron contribution of the non-modelled molecules was subtracted from the diffraction data using the PLATON/SQUEEZE24 procedure. The non-modelled molecules were found to occupy 297 Å3 or 22% of the unit cell. A summary of crystal data is presented in Table 2. Programs PLATON24 and PyMOL were used for analysis of the structure and drawing preparation. Supplementary crystallographic data sets for structures 1a, 1b, 2 (squeezed) and 3 are available through the Cambridge Structural Database with deposition numbers 978263–978265 and 1035947. The second, non-squeezed model of the HL
22b,c), using the WinGX23 program package. All non-hydrogen atoms were refined anisotropically. All hydrogen atoms were assigned calculated positions and allowed to ride on their carrier atoms. There are two versions of structure 2, one with modelled disordered molecules (presented only in the ESI†) and the other one in which the electron contribution of the non-modelled molecules was subtracted from the diffraction data using the PLATON/SQUEEZE24 procedure. The non-modelled molecules were found to occupy 297 Å3 or 22% of the unit cell. A summary of crystal data is presented in Table 2. Programs PLATON24 and PyMOL were used for analysis of the structure and drawing preparation. Supplementary crystallographic data sets for structures 1a, 1b, 2 (squeezed) and 3 are available through the Cambridge Structural Database with deposition numbers 978263–978265 and 1035947. The second, non-squeezed model of the HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br− complex is available by quoting CCDC number 1051952.
Br− complex is available by quoting CCDC number 1051952.
Structural data studies
The study of the thiocyanate complexation modes using selected structural criteria was conducted using the Cambridge Structural Database (CSD 5.24 + 2 updates) for small molecules and Protein Data Bank (PDB) for macromolecules. The obtained data are compared and discussed in terms of geometrical features of our system structures. The available data for chlorate were insufficient to make a reliable conclusion.CSD search
The analysis of the SCN− geometry in supramolecular complexes provided a main set of 238 structures that satisfied the given criteria (3D coordinates determined, not disordered, no errors, not covalently bound or coordinated to the metal ions, not polymeric and with R-factor <0.075; bond lengths and angles were constrained to 1.00–1.25 Å for C–N distance, 1.50–1.75 Å for C–S distance and 170–180° for S–C–N angle), among 85 structures that did not contain metal ions. Considering the importance of SCN−, it is a surprisingly low number of structures, suggesting information deficiency about precise coordinative properties of this ambivalent ion.7 The analyzed hydrogen bonds included N, O and C atoms as donors and S or N(SCN−) atoms as acceptors. In the selected set, 114 structures contained at least one N–H donor group and 92 structures with at least one O–H group. The distances between O and N donor atoms and N(SCN−) atom were limited to 3.6 Å [C(donor)⋯N distance is limited to 3.95 Å] and distances between donor atoms and S(SCN−) atom were limited to 3.9 Å (except for the C–H⋯S bond where the C⋯S distance was limited to 4.0 Å).In all cases, the hydrogen bond angle was in the 90–180° range. In the main set, the N(SCN−) atom was identified as a hydrogen-bonding acceptor in 174 structures. However, in many structures, these interactions overlap and there is a combination of more than one donor group (in 88 cases, the N–H group acts as a donor; 48 structures with the O–H donor group; 120 structures with the C–H donor group). C–H is an exclusive donor for N(SCN−) in 46 (of 120) structures, but only in the structures where no N–H donor group is present. A combination of N–H and C–H donors with a N(SCN−) acceptor atom is present in 82 structures and in combination with O–H group in 5 structures. Combination of O–H and C–H donor groups is present in 19 structures. Out of 177 structures, where X–H⋯SCN− hydrogen bond is observed (X = N, O or C atom), in 108 of 149 of them C–H group is an exclusive donor. In only 60 structures, the donor group for S(SCN−) atom is the N–H group, and in 23 structures, the donor role was given to the O–H group.
Thiocyanate is a known bridging ligand, with N and S groups serving as a donor for coordination to metal cations, resulting in polynuclear coordination compounds. In previous analyses of coordinative abilities of the SCN− anion, the possibility of SCN− as S, N chelating ligand was suggested.7 However, by inspecting the data in our research, no evidence of the chelating S, N donors were found, but many of the structures in our search contain SCN− as a bridging ligand, simultaneously accepting hydrogen bonds by S and N terminal atoms. It could be concluded that the N-terminal side of thiocyanate is in favour of stronger hydrogen-bonding donors, while the S-terminal side is in favour of more hydrophobic interactions with C–H donors. Although simple in geometry and relatively small, this anionic species is far from being a simple building block in the crystal structure architecture.
PDB search
Binding properties of thiocyanate anions in the structures of biological macromolecules were retrieved from the Protein Data Bank. It was found that 217 records contain at least one SCN− molecule per asymmetric unit, out of which 24 structures (with 41 SCN− ions present) were determined using X-ray diffraction with a resolution above 1.5 Å. A set of 34 free thiocyanate ions, not bound to the metal centres, was used for the interaction analysis. RCSB Ligand explorer17 v4.1.0 was used to visualize the interactions and to determine distances between the SCN− ion and the surrounding amino acids/ions/molecules that are within the 4 Å range. The thresholds were as follows: 3.3 Å for the hydrogen bonding interactions, 3.9 Å for the hydrophobic interactions, 3.3 Å for the bridging hydrogen bonding interactions, and 3.5 Å for the metal interactions.In the vast majority of SCN− ions (28), a nitrogen atom was found as an acceptor of strong hydrogen bond donors, water molecules (27 interactions of the N⋯H–O–H type) or amide hydrogen atom (12 interactions of the N⋯H–N type). A total of 47 interactions with the C atom (π-system) was observed in 29 ions: 44 of the C–H⋯C(π) type and 3 of C(π)⋯C(π) type. The remaining ions did not satisfy the threshold range. The sulphur atom binding pocket chemical character was inspected manually in PyMOL.18 In 31 of 34 SCN− ions, the sulphur atom preferred hydrophobic pockets (22 were exclusively hydrophobic), while in only three ions exclusively hydrophilic interactions were observed. The thiocyanate binding behaviour/preference in small molecule crystal structures and that in macromolecule crystal structures are in good agreement. The results of this analysis can offer assistance in thiocyanate modelling in macromolecules.
Acknowledgements
The authors thank the Ministry of Science, Education and Sports of the Republic of Croatia for financial support (grant numbers 119-1191342-1082 and 119-1193079-1084). We are grateful to Prof. Dubravka Matković-Čalogović, Dr. Mirta Rubčić and Mrs. Mia Vrbanac Užarević for critical discussions.Notes and references
- (a) P. A. Gale, Chem. Soc. Rev., 2010, 39, 3746–3771 RSC; (b) A. Basu and G. Das, Dalton Trans., 2012, 41, 10792–10802 RSC; (c) A. Basu and G. Das, Chem. Commun., 2013, 49, 3997–3999 RSC; (d) L. Trembleau, T. A. D. Smith and M. H. Abdelrahman, Chem. Commun., 2013, 49, 5850–5852 RSC; (e) R. Custelcean, Chem. Soc. Rev., 2010, 39, 3675–3685 RSC.
- (a) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS; (b) F. P. Schmidtchen, Chem. Soc. Rev., 2010, 39, 3916–3935 RSC; (c) F. P. Schmidtchen and M. Berger, Chem. Rev., 1997, 97, 1609–1646 CrossRef CAS PubMed; (d) R. Vilar, Struct. Bonding, 2008, 129, 175–206 CrossRef CAS; (e) F. P. Schmidtchen, Top. Curr. Chem., 2005, 255, 1–29 CAS; (f) R. Custelcean and P. Remy, Cryst. Growth Des., 2009, 9, 1985–1989 CrossRef CAS.
- (a) R. Vilar, Angew. Chem., Int. Ed., 2003, 42, 1460–1477 CrossRef CAS PubMed; (b) N. Gimeno and R. Vilar, Coord. Chem. Rev., 2006, 250, 3161–3189 CrossRef CAS PubMed; (c) M. H. Filby and J. W. Steed, Coord. Chem. Rev., 2006, 250, 3200–3218 CrossRef CAS PubMed; (d) M. R. Sambrook, P. D. Beer, J. A. Wisner, R. L. Paul and A. R. Cowley, J. Am. Chem. Soc., 2004, 126, 15364–15365 CrossRef CAS PubMed; (e) M. D. Lankshear and P. D. Beer, Acc. Chem. Res., 2007, 40, 657–668 CrossRef CAS PubMed; (f) R. Sekiya, M. Fukuda and R. Kuroda, J. Am. Chem. Soc., 2012, 134, 10987–10997 CrossRef CAS PubMed.
- K. Ariga and T. Kunitake, Supramolecular Chemistry – Fundamentals and Applications, Springer-Verlag, Berlin, Heidelberg, 2006 Search PubMed.
- (a) A. P. Umali, S. E. LeBouef, R. W. Newberry, S. Kim, L. Tran, W. A. Rome, T. Tian, D. Taing, J. Hong, M. Kwan, H. Heymann and E. V. Anslyn, Chem. Sci., 2011, 2, 439–445 RSC; (b) A. T. Wright and E. A. Anslyn, Chem. Soc. Rev., 2006, 35, 14–28 RSC.
- R. Custelcean, Chem. Commun., 2013, 49, 2173–2182 RSC.
- (a) L. Tchertanov and C. Pascard, Acta Crystallogr., Sect. B: Struct. Sci., 1996, 52, 685–690 CrossRef; (b) L. Tchertanov, Supramol. Chem., 2000, 12, 67–91 CrossRef CAS.
- (a) M. Kabešová, R. Boča, M. Melník, D. Valigura and M. Dunaj-Jurčo, Coord. Chem. Rev., 1995, 140, 115–135 CrossRef; (b) D. A. Buckingham, Coord. Chem. Rev., 1994, 135–136, 587–621 CrossRef.
- (a) H. Zhang, X. Wang, K. Zhang and B. K. Teo, Coord. Chem. Rev., 1999, 183, 157–195 CrossRef CAS; (b) L. Li, L. Zhu, Z.-C. Yue, W.-L. Zhang, B. Zhang, Y.-Y. Niu and H.-W. Hou, CrystEngComm, 2013, 15, 8395–8399 RSC; (c) R. Custelcean, Chem. Commun., 2013, 49, 2173–2182 RSC.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461–466 CrossRef CAS PubMed.
- K. Užarević, I. Đilović, D. Matković-Čalogović, D. Šišak and M. Cindrić, Angew. Chem., Int. Ed., 2008, 47, 7022–7025 CrossRef PubMed.
- (a) K. Užarević, I. Đilović, N. Bregović, V. Tomišić, D. Matković-Čalogović and M. Cindrić, Chem. – Eur. J., 2011, 17, 10889–10897 CrossRef PubMed; (b) N. Bregović, N. Cindro, L. Frkanec, K. Užarević and V. Tomišić, Chem. – Eur. J., 2014, 20, 15863–15871 CrossRef PubMed.
- K. Užarević, I. Halasz, I. Đilović, N. Bregović, M. Rubčić, D. Matković-Čalogović and V. Tomišić, Angew. Chem., Int. Ed., 2013, 52, 5504–5508 CrossRef PubMed.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef PubMed.
- H. M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov and P. E. Bourne, Nucleic Acids Res., 2000, 28, 235–242 CrossRef CAS PubMed.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- J. L. Moreland, A. Gramada, O. V. Buzko, Q. Zhang and P. E. Bourne, BMC Bioinf., 2005, 6, 21 CrossRef PubMed.
- The PyMOL Version 0.99rc6 Schrödinger, 2006 DeLano Scientific LLC.
- A. Mukherjee, S. Tothadi, S. Chakraborty, S. Ganguly and G. R. Desiraju, CrystEngComm, 2013, 15, 4640–4654 RSC.
- (a) S. Liu, S. J. Rettig and C. Orvig, Inorg. Chem., 1991, 30, 4915–4919 CrossRef CAS; (b) P. Gilli, V. Bertolasi, V. Ferretti and G. Gilli, J. Am. Chem. Soc., 2000, 122, 10405–10417 CrossRef CAS.
- Oxford Diffraction, CrysAlis Software System, Version 1.171.31, 2006 Search PubMed.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed; (b) G. M. Sheldrick, SHELXS-2014, Program for Crystal Structure Solution, University of Göttingen, 2014 Search PubMed; (c) G. M. Sheldrick, SHELXL, Version 2014/7, Program for Crystal Structure Refinement, University of Göttingen, 2014 Search PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837 CrossRef CAS.
- (a) P. van der Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194 CrossRef; (b) A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 978263–978265, 1035947 and 1051952. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ce02413c |
| ‡ Current address: McGill University, Department of Chemistry, 801 Sherbrooke St. West, Montreal, QC H3A 0B8, Canada. |
| This journal is © The Royal Society of Chemistry 2015 |