Bis(methyl)(thia/selena)salen Ag(i) complexes: counter-ion induced structural diversity†
Abstract
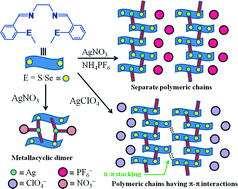
The complexation behaviour of bis(methyl)(thia/selena)salen ligands towards Ag(I) ions in the presence of PF6−, ClO4− and NO3− ions, and the self-assembling of the resultant complexes were explored. Changes in counter anion and coordinating sites (S/Se) led to a structural diversity of complexes as determined using single-crystal X-ray diffraction. The crystallographic study of silver complexes 1 and 2 derived from La and Lb, respectively, in the presence of PF6− ions, revealed the formation of 1D coordination polymers. In the cationic polymer 1, the metal center adopts a distorted square pyramidal geometry where the N2S2 donor set of a ligand molecule forms a basal plane while the S atom from another ligand molecule forms an axial bond, which is responsible for the establishment of the coordination polymer. Unlike complex 1 (with a S-ligating site), complex 2 (with a Se-ligating site) is a polymer with a bimetallic unit where the two metal centers (Ag1 and Ag2) differ in their coordination spheres. In the analogous complexes 3 and 4 involving ClO4− as the counter anion, the polymeric frameworks resemble that of 1 but the existence of π–π interactions between the aromatic rings of the two distinct polymeric chains construct a 2D-sheet, while no direct interaction was observed between the polymeric chains in 1 and 2. The presence of NO3− ions yielded the bimetallic metallacycle 5 possessing a center of inversion. The ligand molecules are coordinated in a tridentate mode and the tetrahedral environment around the silver ion was established with the coordination of a nitrate ion.

 Please wait while we load your content...
Please wait while we load your content...