Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Zero-strain reductive intercalation in a molecular framework†
Joshua A.
Hill
,
Andrew B.
Cairns
 ,
Jared J. K.
Lim
,
Simon J.
Cassidy
,
Simon J.
Clarke
and
Andrew L.
Goodwin
*
,
Jared J. K.
Lim
,
Simon J.
Cassidy
,
Simon J.
Clarke
and
Andrew L.
Goodwin
*
Department of Chemistry, University of Oxford, Inorganic Chemistry Laboratory, South Parks Road, Oxford, OX1 3QR, UK. E-mail: andrew.goodwin@chem.ox.ac.uk; Fax: +44 (0)1865 274690; Tel: +44 (0)1865 272137
First published on 8th January 2015
Abstract
Reductive intercalation of potassium within the molecular framework Ag3[Fe(CN)6] gives rise to a volume strain that is an order of magnitude smaller than is typical for common ion-storage materials. We suggest that framework flexibility might be exploited as a general strategy for reducing cycling strain in battery and ion-storage materials.
One of the central considerations in the development of ion-storage materials is the effect of mass transport on bulk physical properties of the host material.1 The existence of large structural changes on charge or discharge increases the propensity for dislodging, cracking and degradation—all of which reduce battery life and long-term storage capacity.2–4 Upon intercalation most ion-storage media undergo molar volume expansions of between 1 and 10%:4 a response equivalent to a temperature increase of ca. 2000 K.5 The practical consequences of such a large ‘electrochemical shock’ are so severe that much of the field has focussed on developing inventive work-arounds to accommodate volume strain. So, for example, one strategy is that of nanofabrication: by preparing battery materials in the form of nanowires or thin-films it is possible to sustain much larger volume strains, albeit at the expense of storage density.6,7
Ion-storage media that can accommodate Na+ or K+ are increasingly sought-after as battery electrode materials.8,9 These earth-abundant elements could be used in place of lithium for large, static batteries where energy density is much less important compared to mobile applications. One of the challenges in developing electrodes for such batteries is accommodating these much larger ions without drastic volume changes.
We were intrigued by the possibility of exploiting structural flexibility in molecular framework materials as a means of engineering systems with much-reduced intrinsic volume strains. Our particular focus on molecular frameworks has been motivated by the increasingly-apparent predisposition of such materials to anomalous temperature- and pressure-dependent strain behaviour. For example, negative thermal expansion (NTE, volume reduction on heating) is rarely observed in conventional engineering materials10 but is not at all uncommon amongst molecular frameworks;11–13 moreover this NTE effect can be tensioned against the more usual positive thermal expansion contributions of other structural motifs to produce materials with near-zero coefficients of thermal expansion.14 Negative and near-zero compressibilities are also achievable in similar systems.15–17 Such unconventional mechanical responses are rationalised in terms of geometric flexibility: the ease with which molecular frameworks can vary their lattice geometries allows behaviour not observed in conventional materials.18 Given that thermal and pressure-induced strains can be ameliorated by framework flexibility, our hope was that electrochemical strain might also be fundamentally reduced in suitably-chosen molecular frameworks.
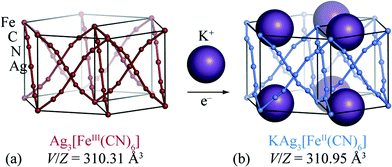
Perhaps the key difficulty faced in developing molecular-framework-based ion-storage materials is that redox chemistry is surprisingly rare for these systems.19 The few molecular frameworks for which bulk insertion has actually been demonstrated include MIL-53(Fe),20,21 lithium isopropoxide-modified magnesium 2,5-dioxido-1,4-benzenedicarboxylate,22 Zn0.5Co0.5(HCOO)3,23 and the Prussian Blue analogue K2Cu[Fe(CN)6].24 Unfortunately, for each of these systems electrochemical strain is not especially reduced relative to “traditional” ion-storage materials such as LixCoO2, and in some cases is actually much larger.25 Encouraged both by a voltammetric study of the [FeII/III(CN)6]4−/3− couple26 and by the discovery of extreme flexibility in silver(I)/gold(I) hexacyanometallates,27 we have chosen to focus on reductive intercalation in silver(I) hexacyanoferrate(III), Ag3[Fe(CN)6] [Fig. 1(a)]. Our X-ray powder diffraction measurements, which are discussed below, reveal that the intrinsic volume strain associated with potassium intercalation in this system is an order of magnitude smaller than for typical ion-storage materials. This ranks Ag3[Fe(CN)6] amongst the few known “zero-strain” insertion compounds (Li4Ti5O12,28 2,6-napth(COOLi)2,29 and Na0.84Ni[Fe(CN)6]0.71 (ref. 30)) in spite of the relatively large ionic radius of potassium [r(K+) = 1.52 Å cf. r(Li+) = 0.90 Å and r(Na+) = 1.16 Å].31
The crystal structure of Ag3[Fe(CN)6] has been reported previously:26,27 octahedral Fe3+ centres are connected via almost-linear dicyanoargentate ions to form three interpenetrating cubic nets [Fig. 1(a)]. The structure has P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 1m crystal symmetry, and includes a set of small vacant channels that run parallel to the trigonal c crystal axis. X-ray powder diffraction patterns for KAg3[Fe(CN)6] have twice been reported,26,32 but to the best of our knowledge its crystal structure has not yet been determined. In contrast, the structure of the Mn analogue KAg3[Mn(CN)6] is known: it is closely related to that of Ag3[Fe(CN)6] except with one half of the channels occupied by K+ cations, lowering the crystal symmetry to P312.16 This close structural similarity suggests that cation insertion within Ag3[Fe(CN)6] framework might be possible via straightforward inclusion of K+ ions within these same channels.
1m crystal symmetry, and includes a set of small vacant channels that run parallel to the trigonal c crystal axis. X-ray powder diffraction patterns for KAg3[Fe(CN)6] have twice been reported,26,32 but to the best of our knowledge its crystal structure has not yet been determined. In contrast, the structure of the Mn analogue KAg3[Mn(CN)6] is known: it is closely related to that of Ag3[Fe(CN)6] except with one half of the channels occupied by K+ cations, lowering the crystal symmetry to P312.16 This close structural similarity suggests that cation insertion within Ag3[Fe(CN)6] framework might be possible via straightforward inclusion of K+ ions within these same channels.
A sample of Ag3[Fe(CN)6], prepared as described previously,27 was reacted at 0 °C with stoichiometric quantities of potassium naphthalenide, using dried THF as solvent and working under a dinitrogen atmosphere on a Schlenk line. The solid product obtained is air-stable and exhibits a qualitatively similar X-ray powder diffraction pattern to the parent Ag3[Fe(CN)6] (as noted in ref. 26) [Fig. 2(a)]. On close inspection, small but meaningful shifts in the Bragg reflection positions and intensities are evident; we note that the difference between the diffraction patterns attributed to Ag3[Fe(CN)6] and KAg3[Fe(CN)6] in ref. 26 map quantitatively onto to those observed here. Rietveld refinement of our powder diffraction data (performed using TOPAS46) using a structural model for KAg3[Fe(CN)6] based on published coordinates for KAg3[Mn(CN)6] (ref. 16) gave an acceptable fit [Fig. 2(a)] and a physically-sensible set of refined parameters (see ESI† for full details). Our refinements indicated a small but significant variation in the lattice parameters (a = 7.0279(5) Å, c = 7.2546(5) Å for the vacant framework and a = 7.06984(23) Å, c = 7.1836(3) Å after intercalation), together with a K-site occupancy of 0.920(13). Infrared absorption spectra measured for starting material and product also reveal the redshift in CN stretching frequencies characteristic of FeIII/FeII reduction [Fig. 2(b)].33,34 The presence of a small quantity of remnant FeIII is suggested by a weak feature in the infrared absorption spectrum near 2170 cm−1 [Fig. 2(b)] and will likely include contributions both from intercalated product (since the K-site occupancy is slightly less than unity) and a small fraction of unreacted Ag3[Fe(CN)6] also evident in the diffraction data (see ESI†). Nevertheless the dominant product is consistent with the formulation K0.92Ag3[Fe(CN)6] (we use KAg3[Fe(CN)6] hereafter for convenience) and with a structural model in which K+ ions are incorporated within one half of the initially-vacant framework channels.
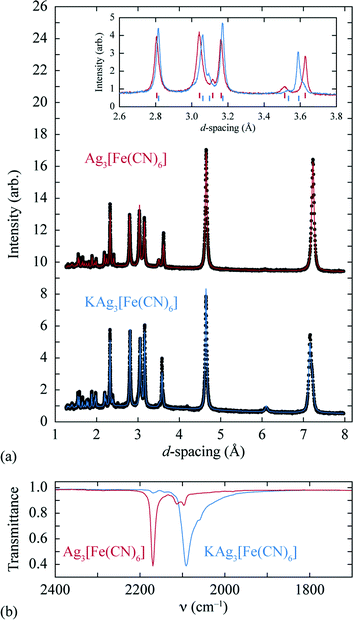 | ||
| Fig. 2 (a) Powder X-ray diffraction patterns and Rietveld fits for the empty (top, Ag3[Fe(CN)6]) and intercalated (bottom, KAg3[Fe(CN)6]) frameworks. A representative section of the diffraction pattern, highlighting the small shifts in peak positions and intensities, is shown in the inset. (b) The change in CN stretching frequencies on intercalation is as expected for FeIII/FeII reduction.33,34 | ||
The volume strain associated with potassium ion intercalation is determined straightforwardly by the variation in lattice constants. We find V = 310.31(5) Å3 for the vacant framework and V = 310.95(3) Å3 after intercalation, corresponding to a volume strain ΔV/V = +0.206(18)%. Table 1 places this value in the context of volume strains measured for a range of topical and well-known ion storage compounds; these data are represented graphically in Fig. 3, where they are distributed according to stored charge density. While this density is (understandably) lower for KAg3[Fe(CN)6] than most Li- and Na-based systems, the volume strain of intercalation is essentially identical to that of the “zero-strain” Li-ion storage material Li2.33Ti1.67O4.37
| Compound | Normalised intercalation volume change (Å3/e−) | Stored charge density (×10−3 e−/Å3) | Intercalation strain, εV = |ΔV/V| (%) | Ref. |
|---|---|---|---|---|
| MgFeSiO4 | 0.75 | 46.86 | 3.53 | 35 |
| LiCoO2 | 0.86 | 9.87 | 0.85 | 25 |
| LiFePO4 | −4.80 | 14.71 | 7.06 | 36 |
| Li 2.33Ti1.67O4 | 0.15 | 13.69 | 0.20 | 37 |
| Li 2[2,6-napth(COOLi)2] | −0.39 | 8.55 | 0.33 | 29 |
| LiC6 | 1.12 | 18.90 | 2.12 | 38 |
| Na 3Mn2(CN)6 | −1.39 | 9.78 | 1.36 | 39 |
| Na 1.5VP4.8F0.7 | −3.23 | 9.50 | 3.07 | 40 |
| Na 2FePO4F | 3.99 | 9.39 | 3.75 | 41 |
| Na 0.84Ni[Fe(CN)6]0.71 | −1.95 | 3.02 | 0.59 | 30 |
| Na 2FeP2O7 | −6.32 | 5.34 | 3.37 | 42 |
| Na 3V2(PO4)3 | −10.08 | 9.08 | 9.15 | 43, 44 |
| Na 2Mn2(CN)6 | 8.50 | 3.24 | 2.75 | 39 |
| K 2Cu[Fe(CN)6] | −9.55 | 2.84 | 2.71 | 24 |
| K 0.92Ag3Fe(CN)6 | −0.70 | 2.96 | 0.21 | This work |
| KC 8 | −41.24 | 14.13 | 58.28 | 45 |
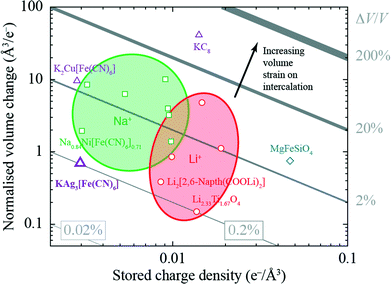 | ||
| Fig. 3 Ashby-type diagram for the ion-storage compounds of Table 1, with data points coloured by intercalant (Mg – blue, Li – red, Na – green, K – purple). The diagonal lines connect points of constant intercalation volume strain εV = ΔV/V. Different applications place different demands on stored charge density (e.g. portability vs. cost), yet in all cases it is desirable to minimise intercalation strain. | ||
The small intercalation strain of KAg3[Fe(CN)6] is explained by the compensating changes in lattice parameters during K+ insertion. On intercalation, the framework expands modestly along the a and b crystal axes in order to widen the channels and accommodate the extra-framework cations. The crucial point is that expansion of the framework in one set of directions results in a contraction along the c axis—the framework behaves much like a three-dimensional “wine-rack” by simply flexing whilst maintaining its basic framework dimensions. So the Fe⋯Fe separation across connected Fe–CN–Ag–NC–Fe linkages (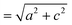 ) changes by just 0.2% on intercalation, whereas the angle between adjacent linkages (given by
) changes by just 0.2% on intercalation, whereas the angle between adjacent linkages (given by 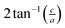 ) changes by 1.0% and hence accounts for most of the (small) strain observed. This behaviour contrasts with the responses observed in ion-storage materials based on layered or dense framework structures. In the former instance (e.g. TiS2), intercalation simply results in increasing interlayer separation; and in the latter (e.g. LiFePO4), the whole lattice expands to accommodate the intercalant. For both types of system the volume strain is inherently large and positive because each of the linear strains is positive. The key advantage offered by molecular frameworks as ion-storage media is that their mechanical responses—whether to variations in temperature or pressure, or to guest inclusion—can be tailored to ensure an expansion in one or more directions is counteracted by a contraction in other directions.13,16,47 Whereas the linear strains induced by changes in temperature and/or pressure can be anomalously large, here we find that the strains can be much smaller than those observed in conventional systems; for example, the linear strain anisotropy Δε = εmax − εmin in the “zero strain” material Li2[2,6-napth(COOLi)2] is 22.70%, but is only 1.58% for KAg3[Fe(CN)6].29
) changes by 1.0% and hence accounts for most of the (small) strain observed. This behaviour contrasts with the responses observed in ion-storage materials based on layered or dense framework structures. In the former instance (e.g. TiS2), intercalation simply results in increasing interlayer separation; and in the latter (e.g. LiFePO4), the whole lattice expands to accommodate the intercalant. For both types of system the volume strain is inherently large and positive because each of the linear strains is positive. The key advantage offered by molecular frameworks as ion-storage media is that their mechanical responses—whether to variations in temperature or pressure, or to guest inclusion—can be tailored to ensure an expansion in one or more directions is counteracted by a contraction in other directions.13,16,47 Whereas the linear strains induced by changes in temperature and/or pressure can be anomalously large, here we find that the strains can be much smaller than those observed in conventional systems; for example, the linear strain anisotropy Δε = εmax − εmin in the “zero strain” material Li2[2,6-napth(COOLi)2] is 22.70%, but is only 1.58% for KAg3[Fe(CN)6].29
In order for this “zero-strain” property of Ag3[Fe(CN)6] to find practical application, it remains to demonstrate the reversibility of the ion storage mechanism we explore here. Similarly, the use of other alkali metals (e.g. Li) and fine-tuning of the redox potentials by substitution at the Fe site are avenues for future work. The key result of this preliminary investigation is to demonstrate how the crucially important problem of intercalation strain might, in principle, be overcome by exploiting geometric flexibility whether such an approach is based on hexacyanometallate chemistry or otherwise.
Acknowledgements
The authors gratefully acknowledge financial support from the E.P.S.R.C. (EP/G004528/2) and the E.R.C. (grant ref.: 279705) to J.A.H., A.B.C., and A.L.G., and from the Diamond Light Source to S.J.C. (Cassidy).References
- Y.-M. Chiang, Science, 2010, 330, 1485–1486 CrossRef CAS PubMed.
- F. Cheng, J. Liang, Z. Tao and J. Chen, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS PubMed.
- F. Yang, J. Power Sources, 2011, 196, 465–469 CrossRef CAS PubMed.
- W. H. Woodford, W. C. Carter and Y.-M. Chiang, Energy Environ. Sci., 2012, 5, 8014–8024 CAS.
- Thermal Expansion of Crystals, ed. R. Krishnan, Pergamon Press, Oxford, 1979, vol. 22 Search PubMed.
- K. T. Nam, D.-W. Kim, P. J. Yoo, C.-Y. Chiang, N. Meethong, P. T. Hammond, Y.-M. Chiang and A. M. Belcher, Science, 2006, 312, 885–888 CrossRef CAS PubMed.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, Nat. Mater., 2010, 9, 353–358 CrossRef CAS PubMed.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS PubMed.
- M. Pasta, C. D. Wessells, N. Liu, J. Nelson, M. T. McDowell, R. A. Huggins, M. F. Toney and Y. Cui, Nat. Commun., 2014, 5, 3001, DOI:10.1038/ncomms4007.
- G. D. Barrera, J. A. O. Bruno, T. H. K. Barron and N. L. Allan, J. Phys.: Condens. Matter, 2005, 17, R217–R252 CrossRef CAS.
- J. L. C. Roswell, E. C. Spencer, J. Eckert, J. A. K. Howard and O. M. Yaghi, Science, 2005, 309, 1350–1354 CrossRef PubMed.
- Y. Wu, A. Kobayashi, G. J. Halder, V. K. Peterson, K. W. Chapman, N. Lock, P. D. Southon and C. J. Kepert, Angew. Chem., 2008, 120, 9061–9064 CrossRef.
- A. L. Goodwin, M. Calleja, M. J. Conterio, M. T. Dove, J. S. O. Evans, D. A. Keen, L. Peters and M. G. Tucker, Science, 2008, 319, 794–797 CrossRef CAS PubMed.
- A. E. Phillips, G. J. Halder, K. W. Chapman, A. L. Goodwin and C. J. Kepert, J. Am. Chem. Soc., 2010, 132, 10–11 CrossRef CAS PubMed.
- A. L. Goodwin, D. A. Keen and M. G. Tucker, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18708–18713 CrossRef CAS PubMed.
- A. B. Cairns, A. L. Thompson, M. G. Tucker, J. Haines and A. L. Goodwin, J. Am. Chem. Soc., 2012, 134, 4454–4456 CrossRef CAS PubMed.
- W. Li, M. R. Probert, M. Kosa, T. D. Bennett, A. Thirumurugan, R. P. Burwood, M. Parinello, J. A. K. Howard and A. K. Cheetham, J. Am. Chem. Soc., 2012, 134, 11940–11943 CrossRef CAS PubMed.
- C. Serre, C. Mellot-Draznieks, S. Surblé, N. Audebrand, Y. Filinchuk and G. Férey, Science, 2007, 315, 1828–1831 CrossRef CAS PubMed.
- C. Combelles, M. B. Yahia, L. Pedesseau and M.-L. Doublet, J. Phys. Chem. C, 2010, 114, 9518–9527 CAS.
- G. Férey, F. Millange, M. Morcrette, C. Serre, M.-L. Doublet, J.-M. Grenèche and J.-M. Tarascon, Angew. Chem., 2007, 119, 3323–3327 CrossRef.
- G. de Combarieu, M. Morcrette, F. Millange, N. Guillou, J. Cabana, C. P. Grey, I. Margiolaki, G. Férey and J.-M. Tarascon, Chem. Mater., 2009, 21, 1602–1611 CrossRef CAS.
- B. M. Wiers, M.-L. Foo, N. P. Balsara and J. R. Long, J. Am. Chem. Soc., 2011, 133, 14522–14525 CrossRef CAS PubMed.
- K. Saravanan, M. Nagarathinam, P. Balaya and J. J. Vittal, J. Mater. Chem., 2010, 20, 8329–8335 RSC.
- C. D. Wessells, R. A. Huggins and Y. Cui, Nat. Commun., 2011, 2, 550 CrossRef PubMed.
- Y. Takahashi, N. Kijima, K. Dokko, M. Nishizawa, I. Uchida and J. Akimoto, J. Solid State Chem., 2007, 180, 313–321 CrossRef CAS PubMed.
- U. Schröder and F. Scholz, Inorg. Chem., 2000, 39, 1006–1015 CrossRef.
- A. L. Goodwin, D. A. Keen, M. G. Tucker, M. T. Dove, L. Peters and J. S. O. Evans, J. Am. Chem. Soc., 2008, 130, 9660–9661 CrossRef CAS PubMed.
- T. Ohzuku, A. Ueda and N. Yamamoto, J. Electrochem. Soc., 1995, 142, 1431–1435 CrossRef CAS PubMed.
- N. Ogihara, T. Yasuda, Y. Kishida, T. Ohsuna, K. Miyamoto and N. Ohba, Angew. Chem., Int. Ed., 2014, 53, 11467–11472 CrossRef CAS PubMed.
- Y. You, X.-L. Wu, Y.-X. Yin and Y.-G. Guo, J. Mater. Chem. A, 2013, 1, 14061–14065 CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- H. Kahlert, Ph.D. thesis, Humboldt-Universität, Berlin, Germany, 1998 Search PubMed.
- A. G. Sharpe, The Chemistry of Cyano Complexes of the Transition Metals, Academic Press, London, 1976 Search PubMed.
- S. B. Moon, A. Xidis and V. D. Neff, J. Phys. Chem., 1993, 97, 1634–1638 CrossRef CAS.
- Y. Orikasa, T. Masese, Y. Koyama, T. Mori, M. Hattori, K. Yamamoto, T. Okado, Z.-D. Huang, T. Minato, C. Tassel, J. Kim, Y. Kobayashi, T. Abe, H. Kageyama and Y. Uchimoto, Sci. Rep., 2014, 4, 5622 Search PubMed.
- A. Yamada, H. Koizumi, N. Sonoyama and R. Kanno, Electrochem. Solid-State Lett., 2005, 8, A409–A413 CrossRef CAS PubMed.
- S. Scharner, W. Weppner and P. Schmid-Beurmann, J. Electrochem. Soc., 1999, 146, 857–861 CrossRef CAS PubMed.
- X.-L. Wang, K. An, L. Cai, Z. Feng, S. E. Nagler, C. Daniel, K. J. Rhodes, A. D. Stoica, H. D. Skorpenske, C. Liang, W. Zhang, J. Kim, Y. Qi and S. J. Harris, Sci. Rep., 2012, 2, 747 Search PubMed.
- H.-W. Lee, R. Y. Wang, M. Pasta, S. Woo Lee, N. Liu and Y. Cui, Nat. Commun., 2014, 5, 5280, DOI:10.1038/ncomms6280.
- Y.-U. Park, D.-H. Seo, H.-S. Kwon, B. Kim, J. Kim, H. Kim, I. Kim, H.-I. Yoo and K. Kang, J. Am. Chem. Soc., 2013, 135, 13870–13878 CrossRef CAS PubMed.
- B. L. Ellis, W. R. M. Makahnouk, Y. Makimura, K. Toghill and L. F. Nazar, Nat. Mater., 2007, 6, 749–753 CrossRef CAS PubMed.
- P. Barpanda, G. Liu, C. D. Ling, M. Tamaru, M. Avdeev, S.-C. Chung, Y. Yamada and A. Yamada, Chem. Mater., 2013, 25, 3480–3487 CrossRef CAS.
- Z. Jian, L. Zhao, H. Pan, Y.-S. Hu, H. Li, W. Chen and L. Chen, Electrochem. Commun., 2012, 14, 86–89 CrossRef CAS PubMed.
- Z. Jian, W. Han, X. Lu, H. Yang, Y.-S. Hu, J. Zhou, Z. Zhou, J. Li, W. Chen, D. Chen and L. Chen, Adv. Energy Mater., 2013, 3, 156–160 CrossRef CAS.
- P. Lagrange, D. Guerard and A. Herold, Ann. Chim., 1978, 3, 143–159 CAS.
- A. A. Coelho, TOPAS-Academic V4.1, Brisbane, 2007 Search PubMed.
- A. L. Goodwin, B. J. Kennedy and C. J. Kepert, J. Am. Chem. Soc., 2009, 131, 6334–6335 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, experimental methods, and sample characterisation; X-ray powder diffraction refinement details. See DOI: 10.1039/c4ce02364a |
| This journal is © The Royal Society of Chemistry 2015 |