Rietveld refinement, cluster modelling, growth mechanism and photoluminescence properties of CaWO4:Eu3+ microcrystals†
Abstract
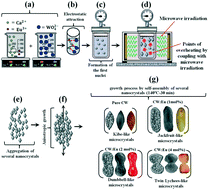
CaWO4:Eu3+ microcrystals (with 0, 1, 2 and 4 mol% Eu3+) were synthesized by a co-precipitation (CP) method and grown in a microwave-assisted hydrothermal (MAH) system at 130 °C for 30 min. X-ray diffraction (XRD), Rietveld refinement, X-ray absorption near edge spectroscopy (XANES), Fourier-transform Raman (FT-Raman) and Fourier-transform infrared (FT-IR) spectroscopy indicated that all of the microcrystals have a scheelite-type tetragonal structure without deleterious phases. Structural refinement data were employed to model the [CaO8], [EuO8] and [WO4] clusters. XANES spectra confirmed that the presence of deltahedral [EuO8] clusters promotes small distortions of neighbouring tetrahedral [WO4] clusters in a global tetragonal lattice. Field emission scanning electron microcopy (FE-SEM) images revealed that the replacement of Ca2+ by Eu3+ ions changed the particles' shapes, resulting in the different morphologies of the microcrystals. UV-vis diffuse reflectance spectra indicated a reduction in the optical band gap with the replacement of Ca2+ by Eu3+ ions. The photoluminescence (PL) properties of the Eu3+ ions in CaWO4 were studied as well as the chromaticity coordinates and lifetimes of these compounds.

 Please wait while we load your content...
Please wait while we load your content...