Open Access Article
Open Access ArticlePore-controlled formation of 0D metal complexes in anionic 3D metal–organic frameworks†
Muwei
Zhang
a,
Mathieu
Bosch
a and
Hong-Cai
Zhou
*ab
aDepartment of Chemistry, Texas A&M University, College Station, Texas 77842, USA. E-mail: zhou@mail.chem.tamu.edu; Fax: +1 979 845 1595; Tel: +1 979 845 4034
bDepartment of Materials Science and Engineering, Texas A&M University, College Station, Texas 77842, USA
First published on 9th December 2014
Abstract
The host–guest chemistry between a series of anionic MOFs and their trapped counterions was investigated by single crystal XRD. The PCN-514 series contains crystallographically identifiable metal complexes trapped in the pores, where their formation is controlled by the size and shape of the MOF pores. A change in the structure and pore size of PCN-518 indicates that the existence of guest molecules may reciprocally affect the formation of host MOFs.
The study of host–guest chemistry has attracted an enormous amount of attention in the past few decades.1–3 Due to their structural diversity and convenient association and dissociation of guest molecules by non-covalent interactions, host–guest compounds can be potentially applied in many areas, including molecular recognition,4–6 reaction capsules,7–9 small molecule structural determination,10–12 sensors,13–15 biological applications,16–18 and many others. Clathrate compounds19–21 are a unique category of host–guest complexes that consist of completely encapsulated guest molecules residing in cages formed by the crystal lattices of host molecules. Due to their crystalline nature, crystallographic tools can be conveniently applied to study their structures for the purpose of understanding the host–guest interactions of these compounds.
The utilization of a host compound with cavities of definite size and shape for selective inclusion of guest molecules is of vital significance for the successful design of host–guest systems.22 Many porous media with intrinsic cavities or openings, including cyclic compounds,23–25 self-assembled molecular cages,26–28 zeolites,29–31 hydrogen-bonded frameworks,32 and metal–organic frameworks (MOFs),10–12,33 have been utilized for the construction of host–guest complexes. Different from traditional porous materials, MOFs exist as 3D coordination networks consisting of metal-containing units and organic linkers.34–36 They have widespread potential applications, such as in energy storage, separation processes, carbon capture, catalysis, luminescent materials, and biomedical applications.37–39 With regard to host–guest chemistry, MOFs appear to be very promising host materials due to their large porosities, customizable cavities, and crystalline nature. For simplicity, in this manuscript, we use the term “MOF clathrates” to describe any MOF-related host–guest compounds in which discrete guest molecules enclosed by MOF cavities are in ordered arrangements throughout the entire framework and can be studied by crystallographic methods. Due to their crystalline nature, MOFs consist of uniform cavities in ordered and periodic arrangements throughout the 3D space with long-range regularity. Upon incorporation of guest molecules that possess appropriate interactions with specific tunable features in MOF pores, they are likely to adopt a particular or preferred conformation and orientation depending on the size, shape, polarity, and alignment of the MOF cavities. This will significantly facilitate the formation of the MOF clathrates. Nevertheless, single-crystal X-ray diffraction (XRD) studies of these compounds are still rare,40 presumably due to the lack of appropriate host–guest interactions between MOFs and the guest molecules. In fact, the guest molecules incorporated in MOFs can rarely be observed by X-ray crystallography.10 In recent years, the Fujita group has reported a series of MOFs as “crystalline sponges”. Due to the high affinity and molecular-recognition ability of the cavities to the target molecules, the molecules absorbed by these MOFs can be regularly placed throughout the MOF lattice, where the molecular structure of these guest molecules, along with their host frameworks, can be conveniently determined by single crystal XRD.10–12 In this work, we report a series of MOF clathrates with incorporated metal complexes as guest molecules. In these MOFs, all of the guest metal complexes are formed in situ and are incorporated into the host framework via Coulombic interactions. Unlike the “crystalline sponge” nature of those reported in Fujita's work, the MOFs utilized in this work serve as “reaction containers”, where the formation of the encapsulated metal complexes is controlled by the size, shape, and electric charge of the cavities of the host MOF. These metal complexes are otherwise not likely to form without the presence of the host framework.
A host MOF that is suitable for the formation of MOF clathrates should possess several distinctive attributes. First, this MOF should possess cavities with an appropriate size. These cavities should be adequately large to contain the guest molecules. Nevertheless, excessively large pores are likely to reduce their host–guest interactions. Second, MOFs with low-symmetry cavities are usually more suitable for obtaining a crystallographic image of the guest molecules. Spherical-shaped cavities are typically not preferred because isotropic host–guest interactions are more likely to result in crystallographic disorders inside the cavities. Low symmetry cavities are more likely to result in a preferred alignment orientation for the guest molecules. Third, suitable host–guest interactions, such as Coulombic attraction, hydrogen bonding, and π⋯π interactions, will facilitate the ordered arrangements of guest molecules inside the MOFs.
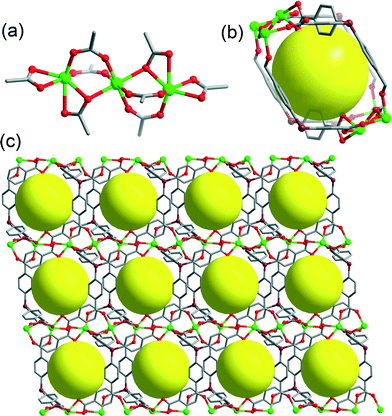
PCN-514 (PCN represents “Porous Coordination Network”) appears to be a promising host for making MOF clathrates.41 It is an ionic framework consisting of an 8-connected trinuclear cadmium cluster (Fig. 1(a)) connected by a tetratopic ligand, TCPS (TCPS = 4,4′,4′′,4′′′-tetrakiscarboxyphenylsilane), in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. It crystallizes in the triclinic space group P
2 ratio. It crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , exhibiting a fluorite (flu) topology42 with a solvent accessible volume43 of 52.40%. It possesses a unique type of cavity with a diameter of 8.4 Å. This cage possesses Ci symmetry with an inversion center located in the geometric center. It is surrounded by four TCPS ligands connected to four trinuclear cadmium clusters (Fig. 1(b) and (c)). It is this cage that controls the formation of a variety of cadmium complexes under different solvothermal conditions. Due to its anionic nature and appropriate pore size, unanchored Cd complexes with coordinating solvents can form inside this cage as counterions of this MOF. The vast majority of previously discovered anionic MOFs typically contain alkylammonium cations that originate from the decomposition of the reaction solvents as their counterions. Examples of positively charged metal-containing coordination complexes formed in situ that fill the MOF cavities as counterions still remain scarce.44,45 It is even rarer for the metal complexes filling the MOF cavities to be crystallographically identified by single crystal XRD studies. In fact, two of these metal-complex-incorporated MOFs were already reported by our group.41 Nevertheless, their host–guest chemistry remains completely undiscussed in the previous manuscript. Herein, we would like to provide a systematic study of the host–guest chemistry of the PCN-514 series and illustrate how the utilization of different solvents results in the formation of different metal complexes with the assistance of MOF cavities.
, exhibiting a fluorite (flu) topology42 with a solvent accessible volume43 of 52.40%. It possesses a unique type of cavity with a diameter of 8.4 Å. This cage possesses Ci symmetry with an inversion center located in the geometric center. It is surrounded by four TCPS ligands connected to four trinuclear cadmium clusters (Fig. 1(b) and (c)). It is this cage that controls the formation of a variety of cadmium complexes under different solvothermal conditions. Due to its anionic nature and appropriate pore size, unanchored Cd complexes with coordinating solvents can form inside this cage as counterions of this MOF. The vast majority of previously discovered anionic MOFs typically contain alkylammonium cations that originate from the decomposition of the reaction solvents as their counterions. Examples of positively charged metal-containing coordination complexes formed in situ that fill the MOF cavities as counterions still remain scarce.44,45 It is even rarer for the metal complexes filling the MOF cavities to be crystallographically identified by single crystal XRD studies. In fact, two of these metal-complex-incorporated MOFs were already reported by our group.41 Nevertheless, their host–guest chemistry remains completely undiscussed in the previous manuscript. Herein, we would like to provide a systematic study of the host–guest chemistry of the PCN-514 series and illustrate how the utilization of different solvents results in the formation of different metal complexes with the assistance of MOF cavities.
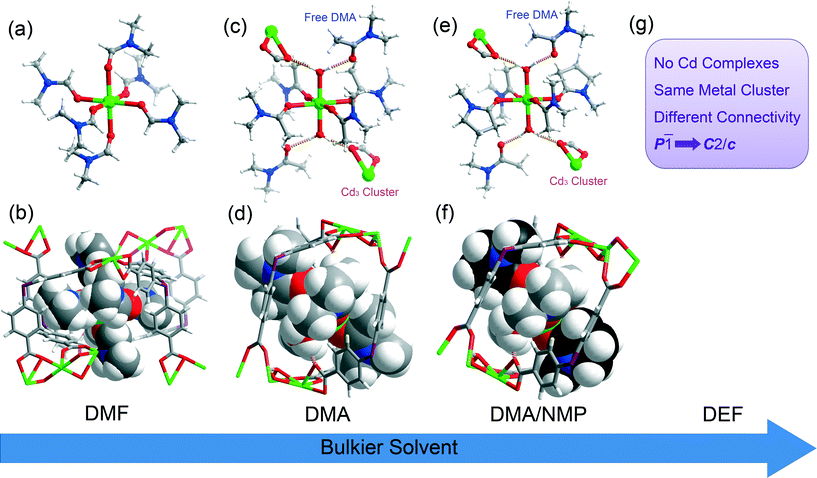
The anionic framework PCN-514 can be generated under a variety of solvothermal conditions. Different metal complexes will form when different solvents are used, while the formation of these trapped metal complexes is controlled by the size and shape of the cavities of this MOF. All of these metal complexes consist of a Cd ion and six coordinating solvent molecules. When bulkier solvents are used, a decreasing number of bulky solvent molecules are observed to coordinate to the metal (see Fig. 2). When DMF (N,N-dimethylformamide) was used, the resulting complex consists of one Cd ion and six DMF molecules, yielding a [Cd(DMF)6]2+ cation residing in the center of each cavity of this framework. When a bulkier solvent, DMA (N,N-dimethylacetamide), was used, the [Cd(DMA)4(H2O)2]2+ cation formed. Each water molecule connects to one free DMA solvent and one Cd3 cluster through hydrogen bonds. When the DMA/NMP (NMP = N-methyl-2-pyrrolidone) mixture was used, formation of [Cd(DMA)2(NMP)2(H2O)2]2+ was observed. In other words, it is the MOF cavities that control the formation, size, and coordination chemistry of the trapped Cd complex. Single crystal XRD studies reveal that all of these Cd complexes possess Ci symmetry with an inversion center located on the Cd metal ion, which is consistent with the symmetry of their host cages.
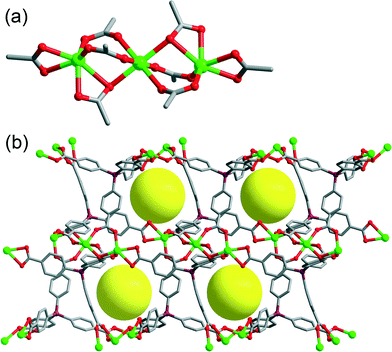
Further exploration of the utilization of bulkier solvents has led to the discovery of a novel MOF, designated as PCN-518. Single crystal XRD studies reveal that it crystallizes in the monoclinic group C2/c. Similar to the PCN-514 series, it is also an anionic MOF that possesses the same Cd clusters (Fig. 1(a)) linked by the TCPS ligand. Nevertheless, even though the cluster of PCN-518 is the same (an 8-connected trinuclear Cd cluster) as that of PCN-514, the connectivity of this cluster is different (Fig. 3(a)). This has resulted in the formation of a topologically different framework, which contains the Cd3 cluster and the tetrahedral ligand in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio to exhibit an alb/P topology.41,46 Interestingly, this framework does not contain any metal complexes as counterions. Instead, it is expected to contain alkylammonium cations trapped in its pores as counterions, which cannot be investigated by crystallographic tools due to their disordered nature. The solvent accessible volume43 of PCN-518 is 48.90%. The diameter of its largest cavities is 6.4 Å (Fig. 3(b)), which is significantly smaller than that of PCN-514. It should be noted that the formation of the PCN-518 framework with trapped Cd–DEF complexes was not observed under solvothermal conditions, presumably because the PCN-518 cavity is too small for any type of Cd–DEF complex to fit into. Without the presence of bulky metal–solvent complexes, the formation of PCN-518 is more energetically favored because the smaller cavities of PCN-518 will significantly enhance the host–guest interactions by increasing the Coulombic attraction between the alkylammonium cations and the host framework. This illustrates a rare example indicating that the existence of guest molecules can reciprocally affect the formation of host frameworks.
2 ratio to exhibit an alb/P topology.41,46 Interestingly, this framework does not contain any metal complexes as counterions. Instead, it is expected to contain alkylammonium cations trapped in its pores as counterions, which cannot be investigated by crystallographic tools due to their disordered nature. The solvent accessible volume43 of PCN-518 is 48.90%. The diameter of its largest cavities is 6.4 Å (Fig. 3(b)), which is significantly smaller than that of PCN-514. It should be noted that the formation of the PCN-518 framework with trapped Cd–DEF complexes was not observed under solvothermal conditions, presumably because the PCN-518 cavity is too small for any type of Cd–DEF complex to fit into. Without the presence of bulky metal–solvent complexes, the formation of PCN-518 is more energetically favored because the smaller cavities of PCN-518 will significantly enhance the host–guest interactions by increasing the Coulombic attraction between the alkylammonium cations and the host framework. This illustrates a rare example indicating that the existence of guest molecules can reciprocally affect the formation of host frameworks.
Conclusions
In summary, we have reported a series of anionic Cd MOFs in this work. Their host–guest chemistry was investigated by single crystal XRD. The PCN-514 series contains metal–solvent complexes that are generated in situ as counterions. These are rarely seen examples of MOF clathrates whose incorporated metal complexes can be crystallographically identified. The formation of these metal complexes is controlled by the size and shape of MOF cavities, where these reactions are otherwise not likely to happen without the host framework. PCN-518 is a topologically different framework without any metal–solvent complexes. It indicates that the formation of host frameworks may be reciprocally controlled by the presence of these metal–solvent complexes as well. Furthermore, this work has provided some insight into the design of MOF-related host–guest systems, where their mutual interactions can be conveniently studied.Acknowledgements
This work was supported as part of the Center for Gas Separations Relevant to Clean Energy Technologies, an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under award number DE-SC0001015, part of the Hydrogen and Fuel Cell Program under award number DE-FC36-07G017033, part of the Methane Opportunities for Vehicular Energy (MOVE) program, an ARPA-E project under award number DE-AR0000249, and part of the Welch Foundation under award number A-1725. M. B. also acknowledges the Texas A&M Graduate Merit Fellowship and Texas Space Grant.Notes and references
- S. Stepanow, M. Lingenfelder, A. Dmitriev, H. Spillmann, E. Delvigne, N. Lin, X. Deng, C. Cai, J. V. Barth and K. Kern, Nat. Mater., 2004, 3, 229–233 CrossRef CAS PubMed.
- G. D. Stucky and J. E. Mac Dougall, Science, 1990, 247, 669–678 CAS.
- D. J. Cram and J. M. Cram, Science, 1974, 183, 803–809 CAS.
- R. M. K. Calderon, J. Valero, B. Grimm, J. de Mendoza and D. M. Guldi, J. Am. Chem. Soc., 2014, 136, 11436–11443 CrossRef CAS PubMed.
- H.-J. Schneider, Angew. Chem., Int. Ed. Engl., 1991, 30, 1417–1436 CrossRef.
- M. Yamamura, T. Saito and T. Nabeshima, J. Am. Chem. Soc., 2014, 136, 14299–14306 CrossRef CAS PubMed.
- M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS PubMed.
- D. H. Leung, D. Fiedler, R. G. Bergman and K. N. Raymond, Angew. Chem., Int. Ed., 2004, 43, 963–966 CrossRef CAS PubMed.
- D. Fiedler, D. H. Leung, R. G. Bergman and K. N. Raymond, Acc. Chem. Res., 2004, 38, 349–358 CrossRef PubMed.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461–466 CrossRef CAS PubMed.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai and M. Fujita, Nat. Protoc., 2014, 9, 246–252 CrossRef CAS PubMed.
- G.-H. Ning, Y. Inokuma and M. Fujita, Chem. – Asian J., 2014, 9, 466–468 CrossRef CAS PubMed.
- F. L. Dickert and P. A. Bauer, Adv. Mater., 1991, 3, 436–438 CrossRef CAS.
- F. L. Dickert and A. Haunschild, Adv. Mater., 1993, 5, 887–895 CrossRef CAS.
- R. V. Slone, K. D. Benkstein, S. Bélanger, J. T. Hupp, I. A. Guzei and A. L. Rheingold, Coord. Chem. Rev., 1998, 171, 221–243 CrossRef CAS.
- T. Douglas and M. Young, Nature, 1998, 393, 152–155 CrossRef CAS PubMed.
- E. A. Meyer, R. K. Castellano and F. Diederich, Angew. Chem., Int. Ed., 2003, 42, 1210–1250 CrossRef CAS PubMed.
- G. C. R. Ellis-Davies, Nat. Methods, 2007, 4, 619–628 CrossRef CAS PubMed.
- J. L. Atwood, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000 Search PubMed.
- S. A. Bourne and M. R. Caira, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2014 Search PubMed.
- T. Iwamoto and D. F. Shriver, Inorg. Chem., 1972, 11, 2570–2572 CrossRef CAS.
- K. Odashima, A. Itai, Y. Iitaka and K. Koga, J. Am. Chem. Soc., 1980, 102, 2504–2505 CrossRef CAS.
- C. A. Stanier, M. J. O'Connell, W. Clegg and H. L. Anderson, Chem. Commun., 2001, 493–494 RSC.
- A.-J. Avestro, D. M. Gardner, N. A. Vermeulen, E. A. Wilson, S. T. Schneebeli, A. C. Whalley, M. E. Belowich, R. Carmieli, M. R. Wasielewski and J. F. Stoddart, Angew. Chem., Int. Ed., 2014, 53, 4442–4449 CrossRef CAS PubMed.
- J. Storsberg and H. Ritter, Macromol. Rapid Commun., 2000, 21, 236–241 CrossRef CAS.
- M. Fujita, Chem. Soc. Rev., 1998, 27, 417–425 RSC.
- C.-Y. Su, Y.-P. Cai, C.-L. Chen, F. Lissner, B.-S. Kang and W. Kaim, Angew. Chem., Int. Ed., 2002, 41, 3371–3375 CrossRef CAS.
- C. J. Bruns, D. Fujita, M. Hoshino, S. Sato, J. F. Stoddart and M. Fujita, J. Am. Chem. Soc., 2014, 136, 12027–12034 CrossRef CAS PubMed.
- G. Calzaferri, S. Huber, H. Maas and C. Minkowski, Angew. Chem., Int. Ed., 2003, 42, 3732–3758 CrossRef CAS PubMed.
- J. A. Incavo and P. K. Dutta, J. Phys. Chem., 1990, 94, 3075–3081 CrossRef CAS.
- S. Megelski and G. Calzaferri, Adv. Funct. Mater., 2001, 11, 277–286 CrossRef CAS.
- M. J. Prakash and S. C. Sevov, Inorg. Chem., 2011, 50, 12739–12746 CrossRef CAS PubMed.
- K. Uemura, S. Kitagawa, K. Fukui and K. Saito, J. Am. Chem. Soc., 2004, 126, 3817–3828 CrossRef CAS PubMed.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS PubMed.
- M. Zhang, M. Bosch, T. Gentle and H.-C. Zhou, CrystEngComm, 2014, 16, 4069–4083 RSC.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- M. Zhang, Z.-Y. Gu, M. Bosch, Z. Perry and H.-C. Zhou, Coord. Chem. Rev., 2014 DOI:10.1016/j.ccr.2014.05.031.
- B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115–1124 CrossRef CAS PubMed.
- M. Zhang, Y.-P. Chen and H.-C. Zhou, CrystEngComm, 2013, 15, 9544–9552 RSC.
- M. Zhang, Y.-P. Chen, M. Bosch, T. Gentle, K. Wang, D. Feng, Z. U. Wang and H.-C. Zhou, Angew. Chem., Int. Ed., 2014, 53, 815–818 CrossRef CAS PubMed.
- A. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2009, 131, 8376–8377 CrossRef CAS PubMed.
- F. Bu and S.-J. Xiao, CrystEngComm, 2010, 12, 3385–3387 RSC.
- L. Wen, P. Cheng and W. Lin, Chem. Sci., 2012, 3, 2288–2292 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures for the ligands, the preparation of the PCN-514 series and PCN-518, and their structure solution and refinement details. Note that the CIF files of PCN-514·DMF (CCDC 942375) and PCN-514·DMA·NMP (CCDC 942374) were already submitted to the CCDC with a previous manuscript (ref. 41). CCDC 1032924 for PCN-514·DMA and 1032925 for PCN-518. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ce02261k |
| This journal is © The Royal Society of Chemistry 2015 |