Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Catalytic enantioselective construction of quaternary stereocenters by direct vinylogous Michael addition of deconjugated butenolides to nitroolefins
Madhu Sudan
Manna
,
Vikas
Kumar
and
Santanu
Mukherjee
*
Department of Organic Chemistry, Indian Institute of Science, Bangalore 560 012, India. E-mail: sm@orgchem.iisc.ernet.in; Fax: +91-80-2360-0529; Tel: +91-80-2293-2850
First published on 16th September 2015
Abstract
Correction for ‘Catalytic enantioselective construction of quaternary stereocenters by direct vinylogous Michael addition of deconjugated butenolides to nitroolefins’ by Madhu Sudan Manna et al., Chem. Commun., 2012, 48, 5193–5195.
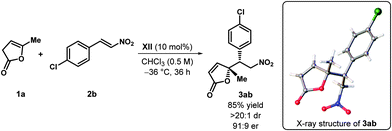
The single crystal X-ray structure of compound 3aa reported in the communication indicated the absolute configuration of the Michael adduct to be (S,S) and the absolute configuration of the remaining Michael adducts were assigned by analogy. However, due to the lack of any heavy atoms in 3aa, an ambiguity regarding the absolute stereochemistry of the Michael adducts remained. To determine the absolute configuration of the Michael adducts unambiguously, we have now synthesized a different Michael adduct 3ab, containing a heavy atom (Cl), catalyzed by our optimized catalyst XII. The single crystal X-ray structure of 3ab revealed its absolute configuration to be (R,R), as shown in the scheme below. As a consequence, the absolute configuration of all the Michael adducts and the compound derived from them (4 in Scheme 1) in the original communication should be opposite.
The corrected structures and data for all the compounds along with the X-ray diffraction data are also included in the revised Supporting Information. CCDC for 3ab is 1420613. The ESI and crystal data have been updated as of 10/09/2015.
These corrections, however, do not alter the conclusion of the original communication.
The graphical abstract image has also been updated as the following:
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2015 |