Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic photoinduced electron transport across a lipid bilayer mediated by a membrane-soluble electron relay†
B.
Limburg
,
E.
Bouwman
and
S.
Bonnet
 *
*
Gorlaeus Laboratories, Leiden Institute of Chemistry, Leiden University, P.O. Box 9502, 2300 RA, Leiden, Netherlands. E-mail: bonnet@chem.leidenuniv.nl
First published on 7th October 2015
Abstract
Unidirectional photocatalytic electron transfer from a hydrophilic electron donor encapsulated in the interior of a liposome, to a hydrophilic electron acceptor on the other side of the membrane, has been achieved using the simple membrane-soluble electron relay 1-methoxy-N-methylphenazinium (MMP+). The total amount of photoproduct (>140 nmol) exceeds the number of moles of MMP+ present (125 nmol), thus showing that the transport of electrons is catalytic.
To accomplish efficient artificial photosynthesis, two complimentary redox reactions must be fuelled by visible light irradiation. In both photoreactions the generation of a long-lived charge-separated species is required, and the greatest challenge in photocatalysis is to overcome charge recombination. In order to do so, the reduced electron acceptor and the oxidized electron donor must be physically separated. In green plants for example such separation is realized by large protein molecules embedded in the thylakoid membrane of chloroplasts. The dissymmetry of biological membranes allows for unidirectional electron transfer to occur from the lumen to the stroma. However, such photocatalytically active protein assemblies are highly complex and fragile, and artificial systems based on simpler, more robust molecules are required for building artificial devices capable of solar fuel production.
Among the numerous tools offered by supramolecular photocatalysis liposomes holds great promise.1 They offer a simple way to organize molecular components in space and mimic biological photosynthesis. By segregating electron donors and acceptors on each side of a lipid membrane, it should be possible to create long-lived charge-separated states that, in combination with two different catalysts, would allow for producing solar fuels.1 Electron transfer across the liposomal membrane to electron acceptors such as quinones,2 Co(bpy)33+,3 or viologens4 has been investigated intensively in the past. However, especially with viologens some ambiguity about the true nature of the charge separation was later revealed.5 It was shown that in those systems electron transport occurred due to diffusion of the reduced electron acceptor through the membrane, after which it remained occluded in the interior of the liposome. Thus, because the electron-transporting molecule never returned to its original location, it was unable to turn over for producing a true catalytic reaction. Up to now, there are only few systems known in the literature that catalytically transport electrons across membranes.2a,d–f,6 However, all of them contain a complicated molecular structure to realize the charge separation across the membrane.
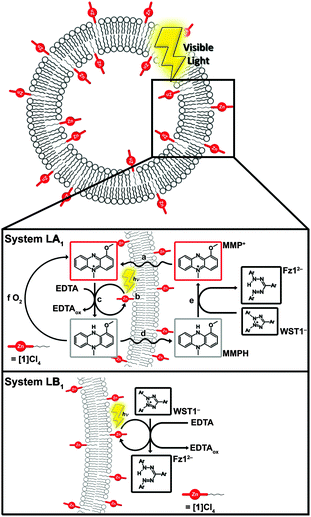
Herein, we report the truly catalytic unidirectional photoinduced electron transfer from the interior of a liposome to its exterior, based largely on commercially available compounds. This study is inspired by the widely used “WST-1” cell-counting assay, in which a water-soluble electron acceptor, the 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium anion (WST1−, Scheme 1), is reduced to the corresponding formazan dye (Fz12−, Scheme 1) by addition of two electrons and one proton. Usually the electrons and protons are generated inside living cells in the form of nicotinamide adenine dinucleotide (NADH), which is too hydrophilic to cross the cell membrane. The WST-1 assay requires the membrane-soluble relay 1-methoxy-N-methylphenazinium cation (MMP+, Scheme 1) to transport the biological reducing equivalents from inside the cell to the WST1− acceptor situated on the other side of the membrane.7 By analogy, we envisioned that biomimetic liposomes containing a water-soluble electron donor in their interior (ethylenediaminetetraacetic acid, H4EDTA) and a photosensitizer in the membrane would be able to unidirectionally transport the photoelectrons to the WST1− molecules located outside the liposomes, provided that MMP+ is added to the system.7 We describe below photocatalytic studies showing that the lipid membrane diminishes product inhibition, while the use of membrane-insoluble electron donors and acceptors allows for avoiding the mechanistic ambiguities generally associated with this type of studies.
Dye-functionalized liposomes were prepared in which the amphiphilic photosensitizer [1]4+ (Scheme 1) was added in the membrane, the electron donor HEDTA3− was placed in the inner water pool of the liposome, and the electron acceptor WST1− was added into the bulk solution, i.e., on the outside of the liposome. The liposome bilayer composition consisted of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1.6 mol% of the alkyl-functionalized zinc porphyrin [1]Cl4, and 1 mol% of the pegylated lipid sodium N-(carbonyl-methoxypolyethylene glycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (NaDSPE-PEG2K) to stabilize the liposome dispersion. The liposomes were prepared by standard extrusion methods in an HEDTA3− buffer at pH = 8, and were subsequently purified by size-exclusion chromatography to remove non-encapsulated HEDTA3− (see ESI†). To this liposomal solution (called hereafter liposome sample LA) was then added an isotonic solution containing the electron acceptor WST1− (as the sodium salt), the electron relay MMP+ (as methylsulfate salt), and ZnSO4. The addition of Zn2+ ions allowed for binding and thus inactivating traces of HEDTA3− that may remain in the bulk aqueous phase after size-exclusion chromatography.4g,h The resulting mixtures, called system LA1 or L2A1 (see compositions in Table 1), were de-aerated and irradiated in the Soret band of [1]4+ (λirr = 449 nm). The excited photosensitizer was assumed to not differ significantly from the well-characterized methyl analogue [ZnTMPyP]4+, i.e., to be of triplet character and to have an oxidation potential of ∼+0.8 V vs. NHE and a reduction potential of ∼−0.45 V vs. NHE.8
| Systema | MMP+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (μM) b (μM) |
Triton X-100 (μM) | O2 | EDTA (mM) | ZnSO4 (mM) | φ 0 (%) |
|---|---|---|---|---|---|---|
| a For the details on the preparation of liposome samples, see ESI. Bulk concentrations before extrusion and/or size exclusion: DPPC (1.04 mM), NaDSPE-PEG2K (10.4 μM), [1]Cl4 (16.7 μM). Final concentration of [1]Cl4 ∼2.1 μM, as determined by UV-vis. b MMP+ added as methylsulfate salt. c For these samples, Na3HEDTA was encapsulated inside the liposomes. To obtain the same osmolality inside and outside the liposomes, all solutions were prepared using isotonic NH4OAc (379 mOsm, pH = 7). d These samples contained twice more liposomes per unit volume than LA1 (DPPC: 2.08 mM, NaDSPE-PEG2K: 20.8 μM, [1]Cl4: 33 μM). | ||||||
| LA1 | 42 | — | — | 5 | 2.4 | |
| LA2 | — | — | — | 5 | — | |
| L2A1 | 42 | — | — | 5 | 2.4 | |
| L2A2 | — | — | — | 5 | — | |
| LA3 | 42 | 0.3 | — | 5 | — | |
| L2A4 | 42 | — | Yes | 5 | — | |
| LB1 | 42 | — | — | 125 | — | 0.90 |
| LB2 | — | — | — | 125 | — | 0.72 |
| LB3 | — | 0.3 | — | 125 | — | 3.8 |
| LB4 | — | — | Yes | 125 | — | 0.84 |
A typical evolution of the UV-vis spectrum of the irradiated dispersions as a function of irradiation time is shown in Fig. 1a. Clearly, a band ascribed to the Fz12− dye arises at λmax = 438 nm as a result of the photoinduced reduction of WST1−.7 The amount of photoproduct, nFz1, determined by the absorbance due to Fz12− (ε438 = 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1),7 was plotted as a function of the number of photons Q(t) absorbed by system since t = 0 (Fig. 1b, L2A1). The slope of this curve at t = 0 was determined to be 1.2(1)% in both LA1 and L2A1. However, as only half of the photosensitizers are assumed to participate in the reaction, only half of the photons are effectively used. The quantum yield for Fz12− formation, φ0, is thus twice higher (2.4(2)%). Dynamic light scattering analysis of the mixture before and after 60 min irradiation gave essentially the same results (Zave = 166 nm, PDI = 0.13 before; Zave = 162 nm, PDI = 0.16 after), indicating that the liposomes stayed intact during photocatalysis. Sample LA1 is thus able to photocatalytically transport the reducing equivalents from inside the liposomes (HEDTA3−) to the outside (WST1−), thus realizing transmembrane charge separation. In L2A1, the twice-higher liposome concentration allowed for observing that more Fz12− was produced during the reaction (141 nmol after 1 hour of irradiation) than the number of moles of MMP+ present (125 nmol) in the system (Fig. 1b), indicating that MMP+ functioned as a true catalyst in this system.
000 M−1 cm−1),7 was plotted as a function of the number of photons Q(t) absorbed by system since t = 0 (Fig. 1b, L2A1). The slope of this curve at t = 0 was determined to be 1.2(1)% in both LA1 and L2A1. However, as only half of the photosensitizers are assumed to participate in the reaction, only half of the photons are effectively used. The quantum yield for Fz12− formation, φ0, is thus twice higher (2.4(2)%). Dynamic light scattering analysis of the mixture before and after 60 min irradiation gave essentially the same results (Zave = 166 nm, PDI = 0.13 before; Zave = 162 nm, PDI = 0.16 after), indicating that the liposomes stayed intact during photocatalysis. Sample LA1 is thus able to photocatalytically transport the reducing equivalents from inside the liposomes (HEDTA3−) to the outside (WST1−), thus realizing transmembrane charge separation. In L2A1, the twice-higher liposome concentration allowed for observing that more Fz12− was produced during the reaction (141 nmol after 1 hour of irradiation) than the number of moles of MMP+ present (125 nmol) in the system (Fig. 1b), indicating that MMP+ functioned as a true catalyst in this system.
 | ||
| Fig. 1 (a) Evolution of the UV-vis spectrum of L2A1 (see Table 1) under blue light irradiation. Spectra were recorded every minute. (b) Photocatalytic formation of Fz12− (nFz1) as a function of the amount of photons Q(t) absorbed by the porphyrin since t = 0. See Table 1 for the composition of systems L2A1 and LB1–4. Conditions: T = 298 K, λirr = 449 nm, Δλ½ = 25 nm, photon flux Φ = 3.3 × 10−8 einstein s−1. Total irradiation time is 30 minutes, or shorter if the absorbance of the solution became higher than A = 3 at 438 nm. | ||
When the electron relay MMP+ was omitted from LA1 or L2A1 (systems LA2 or L2A2, respectively, see Table 1), formation of Fz12− was not observed. Instead the Soret band of the porphyrin bleached to around half of its initial intensity (Fig. S1a, ESI†). It was thus clear that systems LA1 and L2A1 require MMP+ to perform charge separation, and that the electrons cannot be transferred directly from HEDTA3− to WST1− because they are positioned in two different aqueous phases separated by an impermeable lipid membrane. Furthermore, in LA2 and L2A2 only half the porphyrin molecules were bleached, probably those on the inside of the membrane that were photoreduced by HEDTA3−. In system LA3, triton X-100 was added to LA1 prior to the reaction, resulting in disruption of the liposomes and release of HEDTA3− into the bulk solution. In such conditions neither formation of Fz12−, nor bleaching of the porphyrin was observed upon light irradiation. Since the bulk concentration of HEDTA3− was 0.11 mM (see ESI†), i.e. ∼50 times lower than the bulk concentration of Zn2+, the released HEDTA3− was fully bound to the Zn2+ ions thereby losing its ability to donate electrons in system LA3.
In order to investigate in which way physical separation of the electron donor and acceptor by the lipid membrane influences the rate of the photocatalytic reaction, the phototcatalytic system LB1 was developed, which contained both the electron donor and acceptor on the outside of the liposome membrane (Fig. 2, bottom). The liposomes used for these systems, called liposome sample LB, were prepared by standard extrusion methods from a lipid film of DPPC, 1 mol% NaDSPE-PEG2K and 1.6 mol% [1]Cl4 hydrated in aqueous NH4OAc (pH = 7.0). To this liposomal solution were added an isotonic HEDTA3− buffer at pH = 8.0, WST1−, and the electron relay MMP+, to form system LB1 (see Table 1). System LB1 was deaerated and irradiated as described above, which also led to the formation of Fz12−. A much lower initial photoelectron transfer quantum yield φ0 = 0.90(9)% was found for this system compared to LA1, indicating that the physical separation of the electron donor and acceptor in LA1 leads to a more stable separation of charges (Fig. 1b). In system LB1, the photoreduced electron acceptor Fz12− is in the same aqueous phase as the photosensitizer, so that it may also serve as an electron donor and quench the porphyrin excited state, or react with porphyrin cation radicals. Such reactions diminish the overall quantum yield of the photoreaction. When MMP+ is left out from this mixture (system LB2), the reaction is only slightly inhibited (φ0 = 0.72%) compared to LB1 (Fig. 1b), confirming that electron transfer from HEDTA3− to WST1− in LB1 occurs within a single aqueous phase where no membrane needs to be crossed. The addition of triton X-100 to system LB1 (i.e., system LB3) leads to an increase in the efficiency of the photoreaction, confirming that in LA3 triton X-100 quenches the photoreaction solely by disrupting the liposomes.
Many photocatalytic systems are not active in the presence of dioxygen, which would pose a problem in a photocatalytic fuel production system based on water oxidation. Thus, the sensitivity of systems LA1 and LB1 to O2 was investigated. When system LB1 was not deaerated (system LB4) the presence of O2 did not significantly influence the rate of the photoreaction, nor did it cause any induction period common to this type of systems due to the initial competition between WST1− and O2 to accept the first photoelectrons.4g,9 In contrast, when system L2A1 was not deaerated (system L2A4) complete bleaching of the Soret band of the porphyrin was observed and no Fz12− was produced (Fig. S1b, ESI†). The striking difference between systems L2A4 and L2A1 shows that one of the catalytic intermediates in LA1 and L2A1 is unstable in the presence of O2. We hypothesized that such an intermediate would be MMPH, as it plays a key role in the photocatalytic mechanism of system LA1. In a control experiment, system LA1 without WST1− was irradiated in the absence of O2; in this reaction MMPH was produced, which could be detected by UV-vis spectroscopy (Fig. S2, ESI† left). After bubbling air through the solution the reaction was fully reversed: all MMPH was oxidized back to MMP+ (Fig. S2, ESI† right). Thus, photocatalytic electron transfer through the membrane such as that observed in system L2A1 is sensitive to dioxygen because the reduced electron relay is rapidly oxidized by air.
Based on the observations described above the mechanism depicted in Fig. 2 (middle) is proposed for system LA1 and L2A1. First MMP+, introduced in the bulk aqueous phase, diffuses through the membrane into the liposomes (a),7 where it is photoreduced with HEDTA3− (b). This reaction presumably occurs via reductive quenching of *[1]4+ by HEDTA3− (high local concentration, 125 mM), followed by reduction of MMP+ by [1]3+. Oxidative quenching would be thermodynamically feasible (the redox potential of the MMP+/MMPH couple is +0.06 V vs. NHE),10 however kinetically unlikely due to the low concentration of MMP+ (42 μM). MMPH produced inside the liposomes can diffuse through the membrane in absence of O2, thus transporting the electrons and proton out of the liposome (d). Reduction of WST1− by MMPH outside of the liposomes forms Fz12− (e) and regenerates MMP+, as reported for the WST-1 cell counting assay. MMP+ can then diffuse through the membrane again and turnover further. Since the amount of Fz12− produced (e), monitored by UV-vis, exceeds the amount of MMP+ present at the start of the reaction (Fig. 1a), MMP+ must be concomitantly regenerated in the bulk aqueous phase, i.e., MMP+ must be catalytic.
In summary, unlike previous studies in which electron transfer across lipid bilayers occurred concomitantly with the stoichiometric transport and occlusion of the electron acceptor into the membrane, directional electron transfer across a DPPC bilayer is unambiguously demonstrated here by combining a membrane-soluble electron relay, hydrophilic electron donors and acceptors situated inside and outside liposomes, respectively, and a membrane-bound photosensitizer. In this system the MMP+ electron relay truly acts catalytically in absence of oxygen, but under air it is oxidized and cannot fulfill its electron-transporting role. Under inert atmosphere, the actual transport of electrons across the membrane does not limit the reaction rate, as the initial quantum efficiency of the reaction through the membrane (LA1) is three times higher than when the donor and acceptor are both positioned on the same side of the membrane (LB1). Keeping the photoreduced electron acceptor physically separated from the electron donor allows for preventing product inhibition, i.e., quenching of the porphyrin excited states by Fz12−, which would otherwise diminish the quantum efficiency of the photoreduction. Finding a membrane-soluble electron relay that is stable in the presence of dioxygen will be required for the future development of supramolecular photocatalytic systems in which water oxidation and proton reduction catalysts are combined.
Notes and references
- (a) N. Ikuta, S.-y. Takizawa and S. Murata, Photochem. Photobiol. Sci., 2014, 13, 691–702 RSC; (b) M. Hansen, F. Li, L. Sun and B. König, Chem. Sci., 2014, 5, 2683–2687 RSC; (c) S. Troppmann and B. König, Chem. – Eur. J., 2014, 20, 14570–14574 CrossRef CAS PubMed.
- (a) S. Bhosale, A. L. Sisson, P. Talukdar, A. Fürstenberg, N. Banerji, E. Vauthey, G. Bollot, J. Mareda, C. Röger, F. Würthner, N. Sakai and S. Matile, Science, 2006, 313, 84–86 CrossRef CAS PubMed; (b) C. J. Grubb, D. J. Cole-Hamilton and M. K. Whittlesey, J. Chem. Soc., Faraday Trans., 1996, 92, 5005–5012 RSC; (c) Z.-G. Zhao and G. Tollin, Photochem. Photobiol., 1992, 55, 611–619 CrossRef CAS PubMed; (d) M. M. A. Kelson, R. S. Bhosale, K. Ohkubo, L. A. Jones, S. V. Bhosale, A. Gupta, S. Fukuzumi and S. V. Bhosale, Dyes Pigm., 2015, 120, 340–346 CrossRef CAS PubMed; (e) G. Steinberg-Yfrach, P. A. Liddell, S.-C. Hung, A. L. Moore, D. Gust and T. A. Moore, Nature, 1997, 385, 239–241 CrossRef CAS PubMed; (f) G. Steinberg-Yfrach, J. L. Rigaud, E. N. Durantini, A. L. Moore, D. Gust and T. A. Moore, Nature, 1998, 392, 479–482 CrossRef CAS PubMed.
- (a) R. F. Khairutdinov and J. K. Hurst, Nature, 1999, 402, 509–511 CrossRef CAS PubMed; (b) S. V. Lymar, R. F. Khairutdinov, V. A. Soldatenkova and J. K. Hurst, J. Phys. Chem. B, 1998, 102, 2811–2819 CrossRef CAS.
- (a) K. Watanabe, K. Moriya, T. Kouyama, A. Onoda, T. Minatani, S.-Y. Takizawa and S. Murata, J. Photochem. Photobiol., A, 2011, 221, 113–122 CrossRef CAS PubMed; (b) R. Sasaki, Y. Nako and S. Murata, Tetrahedron, 2009, 65, 7364–7371 CrossRef CAS PubMed; (c) T. Mizushima, A. Yoshida, A. Harada, Y. Yoneda, T. Minatani and S. Murata, Org. Biomol. Chem., 2006, 4, 4336–4344 RSC; (d) A. Yoshida, A. Harada, T. Mizushima and S. Murata, Chem. Lett., 2003, 32, 68–69 CrossRef CAS; (e) J. N. Robinson and D. J. Colehamilton, Chem. Soc. Rev., 1991, 20, 49–94 RSC; (f) S. V. Lymar, V. N. Parmon and K. I. Zamaraev, Top. Curr. Chem., 1991, 159, 1–65 CrossRef CAS; (g) W. E. Ford, J. W. Otvos and M. Calvin, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 3590–3593 CrossRef CAS; (h) W. E. Ford, J. W. Otvos and M. Calvin, Nature, 1978, 274, 507–508 CrossRef CAS PubMed.
- (a) L. Hammarström and M. Almgren, J. Phys. Chem., 1995, 99, 11959–11966 CrossRef; (b) L. Hammarström, H. Berglund and M. Almgren, J. Phys. Chem., 1994, 98, 9588–9593 CrossRef; (c) E. R. Kuhn and J. K. Hurst, J. Phys. Chem., 1993, 97, 1712–1721 CrossRef CAS; (d) L. Hammarström, M. Almgren, J. Lind, G. Merenyi, T. Norrby and B. Akermark, J. Phys. Chem., 1993, 97, 10083–10091 CrossRef; (e) L. Hammarström and M. Almgren, Proc. –Indian Acad. Sci., Chem. Sci., 1993, 105, 539–554 CrossRef; (f) S. V. Lymar and J. K. Hurst, J. Am. Chem. Soc., 1992, 114, 9498–9503 CrossRef CAS; (g) L. Hammarström, M. Almgren and T. Norrby, J. Phys. Chem., 1992, 96, 5017–5024 CrossRef; (h) B. C. Patterson, D. H. Thompson and J. K. Hurst, J. Am. Chem. Soc., 1988, 110, 3656–3657 CrossRef CAS; (i) J. K. Hurst and D. H. P. Thompson, J. Membr. Sci., 1986, 28, 3–29 CrossRef CAS; (j) L. Y. C. Lee, J. K. Hurst, M. Politi, K. Kurihara and J. H. Fendler, J. Am. Chem. Soc., 1983, 105, 370–373 CrossRef CAS.
- L. Zhu, R. F. Khairutdinov, J. L. Cape and J. K. Hurst, J. Am. Chem. Soc., 2006, 128, 825–835 CrossRef CAS PubMed.
- M. Ishiyama, M. Shiga, K. Sasamoto, M. Mizoguchi and P. G. He, Chem. Pharm. Bull., 1993, 41, 1118–1122 CrossRef CAS.
- K. Kalyanasundaram and M. Neumann-Spallart, J. Phys. Chem., 1982, 86, 5163–5169 CrossRef CAS.
- B. Limburg, G. Laisné, E. Bouwman and S. Bonnet, Chem. – Eur. J., 2014, 20, 8965–8972 CAS.
- R. Hisada and T. Yagi, J. Biochem., 1977, 82, 1469–1473 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Photocatalytic results in the presence of O2 or in the absence of electron acceptor WST1−. Detailed experimental procedures. See DOI: 10.1039/c5cc07745a |
| This journal is © The Royal Society of Chemistry 2015 |