Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Detecting biologically relevant phosphates with locked salicylaldehyde probes in water†
Namita
Kumari
and
Felix
Zelder
*
Department of Chemistry, University of Zurich, Winterthurerstrasse 190, 8057 Zurich, Switzerland. E-mail: felix.zelder@chem.uzh.ch; Web: http://www.felix-zelder.com Fax: +41 44 635 6803
First published on 2nd October 2015
Abstract
This communication describes a disassembly based approach for the detection of biologically relevant di- and triphosphates in water using locked fluorescent salicylaldehyde probes.
Supramolecular analytical chemistry strives for the development of straightforward approaches for analyte detection in biologically relevant media such as water.1 Over the years, important steps forward, from purely fundamental research towards real-world application, have been achieved in this relatively young research area. In fact, a plethora of indicator systems for detecting anions of biological, medicinal and environmental concerns (e.g. F−, CN−, H2PO4−/HPO42−) have been developed and subsequently improved upon in terms of sensitivity, selectivity, water compatibility and response time.2
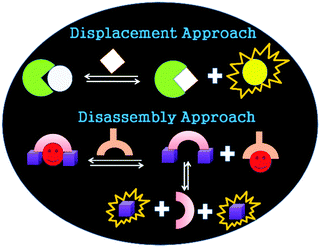
Most of these systems follow one of the three most common approaches: (i) chemosensor, (ii) chemodosimeter or (iii) indicator displacement approach (IDA).3 The working principle of an IDA is shown in Scheme 1. The displacement of a bound indicator from a receptor–indicator complex by a stronger coordinating analyte leads to the displacement of the indicator concomitant with a detectable signal.3
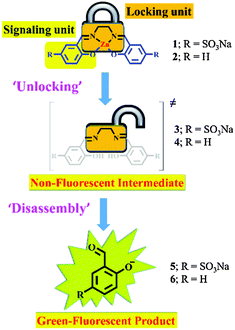
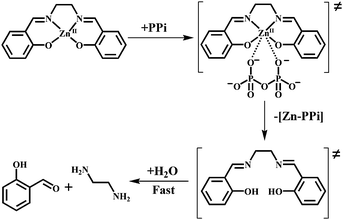
Inspired by this versatile strategy (Scheme 1 top), we present herein an extended version: the disassembly approach (Scheme 1 bottom). In this method, an analyte selectively displaces a metal ion from a metal-chelate containing reagent and the demetallated probe subsequently disassembles into its molecular entities (Scheme 2; ‘disassembly’). One of these molecular building blocks represents the signaling unit of the reaction and hence, the ‘unlocking’ of the reagent leads to a detectable change in the signal (Scheme 1 bottom and Scheme 2).
In this communication, we prove the concept of this sensing strategy by the introduction of a small molecular probe for the fluorometric detection of biologically relevant di-, and triphosphates in water. Our reagent, a zinc salen complex,4 is composed of ZnII, ethylenediamine and two salicylaldehyde subunits. ZnII chelation stabilizes the imine functionalities of the reagent against hydrolysis in water (Scheme 2). Analyte induced metal ion displacement (‘unlocking’; Scheme 2) then activates the reagent for fast hydrolysis into its subunits (‘disassembly’; Scheme 2). The ‘unlocked’ salicylaldehyde subunits produce a ‘turn-on’ green fluorescence at physiological pH and hence act as signaling units in our approach.5 Indeed, the probes were designed to take advantage of both the affinity of ZnII towards phosphate ions6 and the instability of the unlocked, free-base salen ligand in water.7
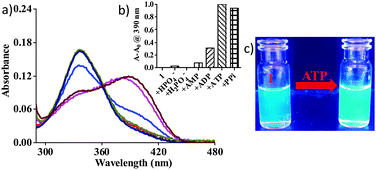
The probes (1, 2) were synthesized as described elsewhere.4 The probes were first tested for the detection of various phosphates and other commonly occurring anions in water at physiological pH 7.4 ([Tris] = 10 mM). Compound 1 with two sulfonate groups was used for all the studies in water. The addition of ATP and PPi (pyrophosphate) to 1 led to a remarkable red shift of 57 nm in the absorption spectrum (Fig. 1). Some small changes were also detected upon addition of ADP. The other phosphates (e.g. H2PO4−, AMP) did not induce any change in absorbance (Fig. 1). None of the other commonly occurring anions (e.g. F−, Cl−, Br−, I−, OAc−, SCN−, NO3−) showed any effect or interference (Fig. S1 and S2, ESI†). Titrations of 1 were therefore performed with both ATP and PPi showing concomitant increases in the absorbance at 380 nm with a gradual decrease at 337 nm (Fig. S3, ESI†).
Sensing of ATP and PPi could also be followed using fluorescence spectroscopy. The addition of ATP or PPi to 1 shifted the fluorescence emission band of 1 from 467 nm (blue) to 490 nm (green) (Fig. 1 and Fig. S4, ESI†). A small change was also observed with ADP. None of the other anions showed any shift in the emission spectra of 1 (Fig. S4, ESI†).
To understand the observed optical changes, the absorption and emission spectra of reaction mixtures of 1 and ATP (or PPi) were compared with the spectra of the free-base salen compound 3 and the salicylaldehyde derivative 5 (Fig. S5, ESI†). Indeed, similarities were observed for the absorption spectrum of derivative 5, but not for that of compound 3 (Fig. S5a, ESI†). Similarly the emission maximum of 5 was identical to that of a mixture of 1 and ATP (or PPi) suggesting the formation of the salicylaldehyde derivative 5 and ethylenediamine during detection (Fig. S5b, ESI†).5 This reaction behaviour was further supported by mass spectral analysis. The spectra of the reaction mixtures displayed an ion at m/z 200.986 that is consistent with the molecular formula of the compound 5 [M − Na]− (Fig. S6 and S7, ESI†). All of these experiments indicate that ATP and PPi displace ZnII from probe 1 generating free-base salen 3 that hydrolyses rapidly in water into its molecular entities, the salicylaldehyde derivative 5 and ethylenediamine. A Job plot analysis indicated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometries for the displacement of ZnII from 1 with PPi, ATP and ADP in water (pH 7.4; Fig. S8, ESI†).8 These experiments suggest that only one equivalent of PPi, ATP or ADP is required to strip ZnII from its salen complex.6 Equilibrium constants for the displacement of ZnII from probe 1 with PPi, ATP and ADP were calculated for 1
1 stoichiometries for the displacement of ZnII from 1 with PPi, ATP and ADP in water (pH 7.4; Fig. S8, ESI†).8 These experiments suggest that only one equivalent of PPi, ATP or ADP is required to strip ZnII from its salen complex.6 Equilibrium constants for the displacement of ZnII from probe 1 with PPi, ATP and ADP were calculated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 interaction using non-linear regression analysis (Fig. S9, ESI†).9 The equilibrium constant values (log
1 interaction using non-linear regression analysis (Fig. S9, ESI†).9 The equilibrium constant values (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K), 4.54 ± 0.04 (PPi), 3.81 ± 0.07 (ATP) and 3.29 ± 0.1 (ADP) indicate stronger interactions between 1 and PPi or ATP compared to ADP at physiological pH.
K), 4.54 ± 0.04 (PPi), 3.81 ± 0.07 (ATP) and 3.29 ± 0.1 (ADP) indicate stronger interactions between 1 and PPi or ATP compared to ADP at physiological pH.
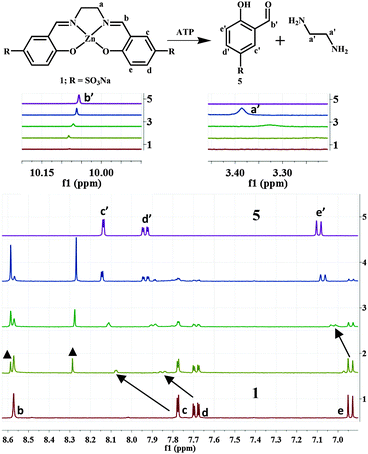
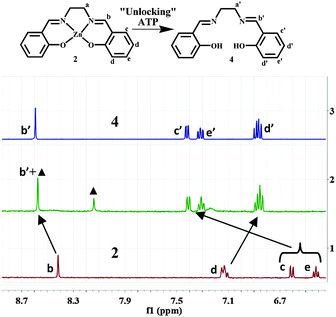
Further insights into the mode of the reaction were obtained using 1H and 31P NMR studies. The addition of ATP to 1 in D2O showed the emergence of new peaks in the 1H NMR spectra concomitant with a gradual decrease in the intensity of existing peaks (b–e) (Fig. 2).
With the increasing addition of ATP, all the proton peaks of 1 disappeared and new peaks (a′–e′) became prevalent. A distinct signal appeared at ∼10.01 ppm. This chemical shift is indicative of the proton of an aldehyde moiety (b′), and hence supports the formation of salicylaldehyde derivative 5 (Fig. 2, line 5). The appearance of new signals at ∼3.3 ppm was also observed. They were assigned to the protons of ‘unmasked’ ethylenediamine, the other product of the disassembly process.
Titrations were also performed with PPi, ADP and AMP (Fig. S10–S12, ESI†). Reactions with the former two anions are comparable to that with ATP. The addition of AMP to 1 did not lead to any changes in the 1H-NMR spectrum. This behaviour indicates that at least two adjacent phosphate subunits are required for demetallation of the reagent. This was further supported by 31P NMR studies of reactions between 1 and ATP, ADP and PPi (Fig. 3 and Fig. S13, ESI†). In this series of reactions, maximal shifts were observed for the β- and γ-phosphorous atoms (β-P, γ-P) of ATP (∼4.0 and 2.5 ppm; Fig. 3), whereas the α-P was almost unaffected (∼0.5 ppm). This behaviour suggests a favoured coordination of the two outer P-atoms (β-, and γ-positions) to the released ZnII ion. The observation is in line with significant shifts of the α- and β-phosphorous atoms of ADP and PPi (∼3.7 and 1.2 ppm; Fig. 3 and Fig. S13, ESI†). No changes were observed for AMP.
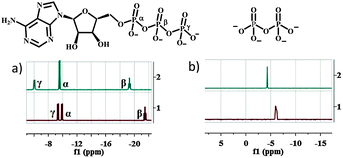 | ||
| Fig. 3 31P NMR spectra of (a) ATP (10 mM) and (b) PPi (10 mM) in the presence (1 eq.; line 2) and absence of compound 1 (line 1) in D2O ([Tris] = 10 mM; pH 7.4). | ||
All of these results strongly suggest that a bidentate chelating phosphate moiety is required for demetallating the reagent and explain nicely the observed selectivity of ATP, ADP and PPi over Pi and AMP.10 We attribute the selectivity pattern to mainly two factors: the chelating effect of the di- and triphosphates as well as their higher anionic charge density of the participating O–P moieties compared to the monophosphates (Pi and AMP). These effects are also expressed in the trend of the equilibria constants of the displacement reaction (PPi > ATP > ADP).
All of these studies have unambiguously proven the disassembly of 1 upon addition of ATP, ADP and PPi into 5 and ethylenediamine in aqueous medium. However, the proposed intermediate of the reaction, the free-base salen ligand 3 was not observed under the reaction conditions. We attributed this behaviour to the fast hydrolysis of the imine functionalities of 3 in water and decided therefore to test the reaction in organic media. All of these studies were performed in an organic medium (dimethyl sulfoxide, DMSO) with probe 2, a ZnII salen complex lacking the sulfonate functionalities. The absorption spectra of 2 were recorded in the presence of different phosphate oxoanions and other potentially interfering anions (e.g. F−, Cl−, Br−, I−, OAc−, SCN−, and NO3−) in DMSO. In agreement with the reaction in water, immediate changes in absorption were only observed for ATP, ADP and PPi (Fig. S14, ESI†). Minor changes were also observed for H2PO4− and AMP, but not for the other tested anions (Fig. S14, ESI†). This time the reaction of 2 with ATP, ADP or PPi resulted in a blue shift (∼40 nm) from 357 nm to 317 nm. This reaction was assigned to the formation of free-base salen 4 by comparing its UV-vis spectra to that of the pure compound (Fig. S15, ESI†). The displacement reaction of 2 with ATP (PPi) in DMSO was also followed by mass spectrometry, suggesting the formation of the (demetallated) free-base salen compound 4 (Fig. S16 and S17, ESI†).
Further evidence for the displacement of ZnII was given by 1H NMR studies. The titration of 2 with ATP in DMSO-d6 resulted in downfield shifts for Ha (0.2 ppm), Hb (0.2 ppm), Hc (0.8 ppm) and He (0.7 ppm), whereas Hd was shifted upfield (0.3 ppm; Fig. 4). Selective demetallation of 2 (‘unlocking’: Scheme 2) with ATP in DMSO-d6 was then unambiguously confirmed by comparing the 1H NMR spectra of a mixture of 2 and ATP and free-base salen 4 (Fig. 4 and Fig. S18, ESI†). Again, the two NMR spectra are almost identical. 1H NMR titrations of 2 with PPi and ADP gave similar results (Fig. S19 and S20, ESI†). This confirms that the ZnII-containing reagent acts as an indicator for biologically relevant phosphate ions (ATP, ADP and PPi) in DMSO following a displacement approach.
For testing the hydrolysis of 4 in water, we subsequently added D2O (4–12%) to this reaction mixture. Indeed, the formation of salicylaldehyde 6 was unambiguously observed using 1H-NMR spectroscopy (Fig. S21, ESI†). Thus the results clearly support our hypothesis (Scheme 3) that the ZnII-containing reagent is first demetallated with di-and triphosphates (ATP, ADP and PPi) to the free-base salen ligand which is then rapidly hydrolysed into its molecular subunits, salicylaldehyde and ethylenediamine.
Rapid analyte detection is observed with this new method. Indeed, quantitative optical changes were observed instantaneously after addition of ATP or PPi (10 eq.) to the reagent (1; 20 μM) (Fig. S22, ESI†). Nevertheless, the current reagent still has some limitations due to its relatively low stability in buffered medium (Fig. S22, ESI†). Future improvements by structural modifications of the complexes are therefore required. Metal-sal(oph)en complexes offer ample opportunities for this purpose and may also lead to the discovery of new probes with altered selectivity.
The sensing of biologically relevant phosphate anions with metal complexes is common.11 However, only two studies have been reported so far on ZnII-sal(oph)en-based receptors for the detection of nucleoside phosphates.12 These compounds elegantly combined ZnII–phosphate coordination with π–π stacking or hydrogen bonding interactions for the molecular recognition of adenosine di- and tri-phosphates. To the best of our knowledge, a ZnII-based reagent for the detection of biologically relevant phosphates following a displacement strategy has not been reported so far.
In summary, we describe herein the disassembly approach as a novel strategy for analyte detection. In particular, we introduced as a proof-of-concept, a ZnII-salen complex as a locked fluorescent salicylaldehyde probe for the “turn-on” detection of biologically relevant di-and triphosphates under physiological conditions. It is anticipated that this strategy is not limited to the current examples and will find other applications in the near future.
A post-doctoral fellowship to N.K. by the ‘Forschungskredit (grant number FK-14-107)’ of the University of Zurich is gratefully acknowledged. Prof. H. J. Jessen is kindly acknowledged for a generous gift of tetrabutylammonium salts of ATP, ADP and AMP. Mr U. Stalder and Dr T. Fox are kindly acknowledged for their help with MS and NMR studies.
Notes and references
- (a) A. P. Davis, S. Kubik and A. Dalla Cort, Org. Biomol. Chem., 2015, 13, 2499 RSC; (b) G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, Angew. Chem., Int. Ed., 2007, 46, 2366 CrossRef CAS PubMed; (c) E. V. Anslyn, J. Org. Chem., 2007, 72, 687 CrossRef CAS PubMed.
- (a) J. Wu, B. Kwon, W. Liu, E. V. Anslyn, P. Wang and J. S. Kim, Chem. Rev., 2015, 115, 7893 CrossRef CAS PubMed; (b) N. Kumari, N. Dey and S. Bhattacharya, Analyst, 2014, 139, 2370 RSC; (c) N. Kumari, S. Jha and S. Bhattacharya, Chem. – Asian J., 2014, 9, 830 CrossRef CAS PubMed; (d) C. Männel-Croisé and F. Zelder, ACS Appl. Mater. Interfaces, 2012, 4, 725 CrossRef PubMed; (e) N. Kumari, S. Jha and S. Bhattacharya, Chem. – Asian J., 2012, 7, 2805 CrossRef CAS PubMed; (f) C. Männel-Croisé and F. Zelder, Anal. Methods, 2012, 4, 2632 RSC; (g) N. Kumari, S. Jha and S. Bhattacharya, J. Org. Chem., 2011, 76, 8215 CrossRef CAS PubMed.
- (a) M. E. Moragues, R. Martínez-Máñez and F. Sancenón, Chem. Soc. Rev., 2011, 40, 2593 RSC; (b) Z. Xu, X. Chen, H. N. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 127 RSC; (c) R. Martínez-Máñez and F. Sancenón, Coord. Chem. Rev., 2006, 250, 3081 CrossRef PubMed; (d) B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118 CrossRef CAS PubMed; (e) A. Buryak and K. Severin, J. Am. Chem. Soc., 2005, 127, 3700 CrossRef CAS PubMed; (f) Z. Zhong and E. V. Anslyn, J. Am. Chem. Soc., 2002, 124, 9014 CrossRef CAS PubMed; (g) L. Fabbrizzi, N. Marcotte, F. Stomeo and A. Taglietti, Angew. Chem., Int. Ed., 2002, 41, 3811 CrossRef CAS; (h) L. Fabbrizzi, A. Leone and A. Taglietti, Angew. Chem., Int. Ed., 2001, 40, 3066 CrossRef CAS; (i) S. L. Wiskur and E. V. Anslyn, J. Am. Chem. Soc., 2001, 123, 10109 CrossRef CAS.
- (a) E. Delahaye, M. Diop, R. Welter, M. Boero, C. Massobrio, P. Rabu and G. Rogez, Eur. J. Inorg. Chem., 2010, 4450 CrossRef CAS PubMed; (b) A. Dalla Cort, L. Mandolini, C. Pasquini, K. Rissanen, L. Russo and L. Schiaffino, New J. Chem., 2007, 31, 1633 RSC.
- S. Hisaindee, O. Zahid, M. A. Meetani and J. Graham, J. Fluoresc., 2012, 22, 677 CrossRef CAS PubMed.
- H. Sigel and R. Griesser, Chem. Soc. Rev., 2005, 34, 875 RSC.
- O. G. Tsay, S. T. Manjare, H. Kim, K. M. Lee, Y. S. Lee and D. G. Churchill, Inorg. Chem., 2013, 52, 10052 CrossRef CAS PubMed.
- P. Job, Ann. Chim. Appl., 1928, 9, 113 CAS.
- (a) A. S. Rao, D. Kim, H. Nam, H. Jo, K. H. Kim, C. Ban and K. H. Ahn, Chem. Commun., 2012, 48, 3206 RSC; (b) M. J. McDonough, A. J. Reynolds, W. Y. G. Lee and K. A. Jolliffe, Chem. Commun., 2006, 2971 RSC; (c) S. Jordan, V. Koprivica, R. Chen, K. Tottori, T. Kikuchi and C. A. Altar, Eur. J. Pharmacol., 2002, 441, 137 CrossRef CAS; (d) H. J. Motulsky and A. Christopoulos, Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting, GraphPad Software Inc., San Diego, CA, 2003 Search PubMed.
- (a) H. N. Lee, K. M. K. Swamy, S. K. Kim, J.-Y. Kwon, Y. Kim, S.-J. Kim, Y. J. Yoon and J. Yoon, Org. Lett., 2007, 9, 243 CrossRef CAS PubMed; (b) D. H. Lee, S. Y. Kim and J.-I. Hong, Angew. Chem., Int. Ed., 2004, 43, 4777 CrossRef CAS PubMed.
- (a) S. Lee, K. K. Y. Yuen, K. A. Jolliffe and J. Yoon, Chem. Soc. Rev., 2015, 44, 1749 RSC; (b) E. A. Kataev and T. A. Shumilova, Molecules, 2015, 20, 3354 CrossRef PubMed; (c) A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603 CrossRef CAS PubMed and references therein; (d) E. Kataev, R. Arnold, T. Rüffer and H. Lang, Inorg. Chem., 2012, 51, 7948 CrossRef CAS PubMed.
- (a) M. Strianese, S. Milione, A. Maranzana, A. Grassi and C. Pellecchia, Chem. Commun., 2012, 48, 11419 RSC; (b) M. Cano, L. Rodríguez, J. C. Lima, F. Pina, A. Dalla Cort, C. Pasquini and L. Schiaffino, Inorg. Chem., 2009, 48, 6229 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional schemes and figures. See DOI: 10.1039/c5cc07413d |
| This journal is © The Royal Society of Chemistry 2015 |