Open Access Article
Open Access ArticleEfficient singlet oxygen generation from sugar pendant C60 derivatives for photodynamic therapy†
Shigenobu
Yano
*ab,
Masami
Naemura
c,
Akio
Toshimitsu
c,
Motofusa
Akiyama
d,
Atsushi
Ikeda
e,
Jun-ichi
Kikuchi
a,
Xiande
Shen
f,
Qian
Duan
f,
Atsushi
Narumi
g,
Masahiro
Inoue
h,
Kei
Ohkubo
ij and
Shunichi
Fukuzumi
*ijk
aGraduate School of Material Science, Nara Institute of Science and Technology, 8916-5, Takayama-cho, Ikoma, Nara 630-0192, Japan. E-mail: yano-shigenobu@ms.naist.jp
bOffice of Society-Academia Collaboration for Innovation, Kyoto University, Kyoto-daigaku Katsura, Nishikyo-ku, Kyoto 615-8520, Japan
cDepartment of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto-daigaku Katsura, Nishikyo-ku, Kyoto 615-8520, Japan
dDepartment of Applied Chemistry, Faculty of Science and Engineering, Chuo University, 1-13-27 Kasuga, Bunkyo-ku, Tokyo 112-8551, Japan
eDepartment of Applied Chemistry, Graduate School of Engineering, Hiroshima University, 1-4-1, Kagamiyama, Higashi-Hiroshima 739-8527, Japan
fSchool of Materials Science and Engineering, Changchun University of Science and Technology, Changchun, 130022, China
gDepartment of Polymer Science and Engineering, Graduate School of Science and Engineering, Yamagata University, Jonan 4-3-16, Yonezawa 992-8510, Japan
hDepartment of Biochemistry, Osaka Medical Centre for Cancer and Cardiovascular Diseases, 1-3-3 Nakamichi, Higashinari-ku, Osaka 537-8511, Japan
iDepartment of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA and SENTAN, Japan Science and Technology Agency (JST), 2-1 Yamada-oka, Suita, Osaka 565-0871, Japan. E-mail: fukuzumi@chem.eng.osaka-u.ac.jp
jDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul 120-750, Korea
kFaculty of Science and Engineering, Meijo University, ALCA and SENTAN, Japan Science and Technology Agency (JST), Nagoya, Aichi 468-0073, Japan
First published on 17th September 2015
Abstract
The amidation reaction between C60 with an activated ester group (1) and acetylated Glc (AcGlc) with an amino group (2) was performed to yield the target AcGlc-pendant C60 compound (3). The water soluble deacetylated compound, Glc-pendant C60 compound (4), exhibited high photocytotoxicity against HeLa cells due to the more efficient singlet oxygen generation as compared with that of Glc-pendant azafulleroids.
Photodynamic action is initiated by the absorption of a photon followed by many competing radiative and nonradiative reactions, which ultimately result in the oxidation and degradation of vital biomolecules. Molecular oxygen plays a key role in the propagation of the initial molecular damage, resulting in vascular collapse, tissue destruction, and cell death. Photodynamic therapy (PDT) has attracted much attention as a less invasive method for treating cancer, because PDT induces tumour cell necrosis and/or apoptosis by producing reactive oxygen species (ROS) through an activated photosensitiser (PS) that accumulates specifically in the tumor.1,2 The potential applications of fullerenes and their derivatives have increased in recent years, particularly in the fields of biology and medicine, where they can be used as DNA photo-cleaving agents, anti-HIV protease inhibitors, antibacterial agents and PSs for PDT.3–10 Although carbohydrates play essential roles in biological systems, their usage in fullerene-based PDT has yet to be fully explored. We have previously reported that sugar-pendant [60]fullerene (C60) derivatives prepared from carbohydrate linked azides exhibited the singlet oxygen producing ability in DMSO to demonstrate the carbohydrate-dependent photocytotoxicity against the HeLa cells.11,12 However, the singlet oxygen yields of these D-glucose (Glc) pendant azafulleroids were significantly smaller than that of pristine C60.11 It is highly desired to develop water soluble sugar-pendant C60 compounds without decreasing the singlet oxygen yield upon photoirradiation.
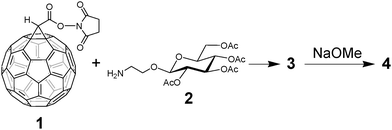
We report herein the synthesis of a new family of Glc-pendant C60 compounds, in which sugar and photosensitive units are connected via the cyclopropane bridged carbon-linkage to retain the conjugate properties of pristine C60, such as 2′-(2′,3′,4′,6′-O-tetraacetyl-β-D-glucopyranosyl)ethyl 3′H-cyclopropa[1,9](C60-Ih)[5,6]-fullereno-3′-carboxylic amide (3) and 2′-(β-D-glucopyranosyl)ethyl 3′H-cyclopropa[1,9](C60-Ih)[5,6]fullereno-3′-carboxylic amide (4) (Chart 1). The photocytotoxicities against HeLa cells and photophysical processes of 3 and 4 were compared with previously prepared Glc-pendant azafulleroids, such as 1a-aza-1a-[2′-(2′,3′,4′,6′-O-tetraacetyl-β-D-glucopyranosyl)ethyl]-1(2)a-homo(C60-Ih)[5,6]fullerene (5) and 1a-aza-1a-[2′-(β-D-glucopyranosyl)-ethyl]-1(2)a-homo(C60-Ih)[5,6]-fullerene (6) (Chart 1).11
 | ||
| Chart 1 Structures of Glc-pendant C60 compounds 3 and 4 in this study and previously prepared Glc-pendant azafulleroids 5 and 6. | ||
Scheme 1 shows the synthetic procedure of 3 and 4. 3′H-Cyclopropa[1,9][5,6]fullereno-C60-Ih-3′-carboxylic acid N-hydroxysuccinimide ester (1) was prepared from the corresponding C60 acid derivative, 3′H-cyclopropa[1,9][5,6]fullereno-C60-Ih-3′-carboxylic acid,13 and N-hydroxysuccinimide (NHS). 2-Aminoethyl 2,3,4,6-O-tetraacetyl-β-D-glucopyranoside (2) was prepared by the similar procedure to that described in the literature,14 in which Pd/C was used in place of the Lindlar catalyst. We performed the amidation reaction between 1 and 2 in dry CHCl3, followed by the purification using silica gel column chromatography (eluent: CHCl3/MeOH = 50/1, Rf = 0.20) to afford 3 as a brown solid. Subsequently, 3 was treated with sodium methoxide in dry THF to quantitatively afford the final target compound 4. The detailed procedures are described in the ESI.†
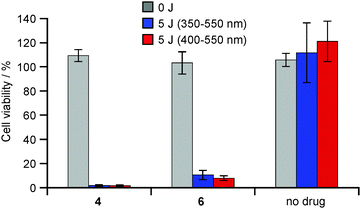
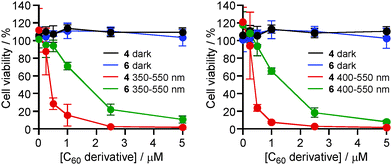
The photodynamic activities were evaluated using human cervical cancer HeLa cells, to compare between two kinds of Glc-pendant C60 derivatives (4 and 6) (Fig. 1). Following incubation with the respective C60 derivatives, the cells were exposed to light with wavelengths between 350 and 550 nm. The results showed that no samples had dark toxicity, even at the highest concentrations used. On the other hand, the viabilities of HeLa cells were reduced depending on photoirradiation. These photodynamic activities of Glc-pendant C60 derivatives were drug dose-dependent and the medium inhibitory concentrations (IC50 values) were estimated to be ca. 0.4 μM for 4 and 1.6 μM for 6. Furthermore, similar tendencies regarding the photodynamic activities were observed even when the light wavelengths were changed to 400–550 nm at the same light dose (Fig. 2). The IC50 values were estimated to be ca. 0.4 μM for 4 and 1.5 μM for 6 (Table S1 in the ESI†). These data indicate that the photodynamic activity of 4 is about 4 times higher than that of 6.
In order to rationalize such drastic differences in the photodynamic activity, the singlet oxygen generation properties were characterized for 3 and 5. These samples produced singlet oxygen in the oxygen-saturated C6D6 solutions by photoirradiation, which could be quantified from the peak area due to the singlet oxygen phosphorescence at 1270 nm (Fig. S1 in the ESI†).15,16 The quantum yield of singlet oxygen generation from 3 was determined to be 0.61, which is much larger than that from 5 (0.22). The larger singlet oxygen yield from 3 results from the efficient formation of the triplet excited state of the C60 moiety, which has the much longer lifetime as compared with that derived from 5. This was also supported by femtosecond laser-induced transient absorption measurements of 4 and 6. Femtosecond laser excitation of a deaerated DMSO solution of 6 at 355 nm resulted in the instant observation of a transient absorption band at 700 nm due to the triplet excited state of C60, which increased at 3000 ps as shown in Fig. 3a. In contrast to the case of 4, femtosecond laser excitation of a deaerated DMSO solution of 6 at 355 nm resulted in the formation of C60˙− as revealed by the transient absorption at 1000 nm,17 together with the triplet excited state of C60 at 700 nm, which decayed significantly at 3000 ps as shown in Fig. 3b. Similar results were obtained for the acetylated compounds of 3 and 5 (Fig. S2 and S3 in the ESI†).
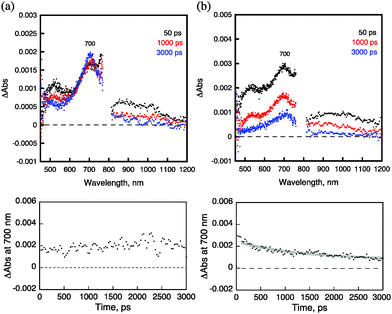 | ||
| Fig. 3 Transient absorption spectra (upper panels) and time profiles (lower panels) at 700 nm of (a) 4 and (b) 6 in deaerated DMSO taken 50, 1000, and 3000 ps after laser excitation at 355 nm. | ||
The formation of the triplet excited state of C60 without the formation of C60˙− of 4 was confirmed by nanosecond laser transient absorption spectra, whereas the transient absorption spectra of C60˙− were observed for 6 as shown in Fig. S4 (ESI†), where the formation of the triplet excited state of C60 of 3 and C60˙− of 5 are also observed. The formation of C60˙− of 5 and 6 was also confirmed by the EPR spectra recorded after photoirradiation at 143 K as shown in Fig. S5 (ESI†),18 where only a weak EPR signal due to C60˙− was observed for 3 and 4.
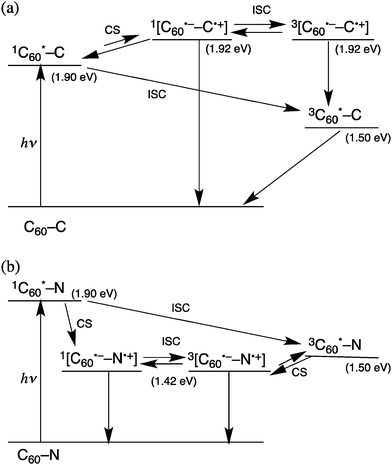
No observation of C60˙− (Fig. 3a) and the slow formation of the triplet excited state of C60 (Fig. 3a) indicate that no photoinduced electron transfer occurs from the Glc moiety to the singlet excited state and the triplet excited state of the C60 moiety. This is verified by the determination of the redox potentials of 3–6 by cyclic voltammetry and second harmonic ac voltammetry (SHACV)19 as shown in Fig. S6–S9 in the ESI.† The one-electron oxidation potential of the Glc moiety of 4 was determined to be 1.28 V vs. SCE by SHACV, whereas the one electron reduction potential of the C60 moiety of 4 was determined to be −0.64 V vs. SCE by CV. Because the singlet excited state energy of the C60 moiety of 4 was determined to be 1.92 eV, the free energy change of electron transfer from the Glc moiety of 4 to the singlet excited state of C60 is evaluated to be 0.02 eV, which means that the electron transfer is slightly endergonic as shown in Scheme 2a. In such a case, electron transfer from the Glc moiety of 4 to the singlet excited state of C60 may be followed by faster back electron transfer to the singlet excited state of C60 or the triplet excited state of C60 without observation of C60˙−.
The occurrence of electron transfer from the Glc moiety of 6 to the singlet excited state of C60 (Fig. 3b) is verified by the lower oxidation potential of 6 as compared with that of 4 because of the N-linkage in 6 (Fig. S9 in the ESI†). In this case, the free energy change of electron transfer from the Glc moiety of 6 to the singlet excited state of C60 is evaluated to be −0.40 eV, which means the electron transfer is highly exergonic (Scheme 2b). Because the energy of the charge-separated state (1.50 eV) is the same as the energy of the triplet excited state of C60, the triplet excited state of C60 is produced via the intersystem crossing of the charge-separated state (Scheme 2b). However, the triplet excited state of C60 of 6 decays via the charge-separated state with the faster rate than the case of 4.
In conclusion, the D-glucose (Glc) pendant C60 compound (4) exhibited significantly higher photocytotoxicity against HeLa cells than the corresponding azafulleroid, because of no involvement of the charge-separated state in the decay of the triplet excited sate of the C60 moiety of 4, which resulted in the higher yield of singlet oxygen. The present study provides a rational design of water soluble sugar-pendant C60 derivatives for more efficient PDT.
This work was supported by JSPS KAKENHI grant numbers 19350031, 23590923, 25288028, 25288037, 26620154, 26288037, the Japan–German Exchange Program supported by the JSPS and the Deutsche Forschungsgemeinschaft (DFG), and by the Japan Advanced Molecular Imaging Program (J-AMP) of the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant-in-Aid of Kyoto University for Young Scientists, grant from the San-Ei-Gen Foundation for chemical research, and by ALCA and SENTAN projects from JST, Japan.
Notes and references
- (a) H.-Q. Peng, L.-Y. Niu, Y.-Z. Chen, L.-Z. Wu, X.-H. Tung and Q.-Z. Yang, Chem. Rev., 2015, 115, 7502–7542 CrossRef CAS PubMed; (b) L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- (a) F. Anzengruber, P. Avci, L. F. de Freitas and M. R. Hamblin, Photochem. Photobiol. Sci., 2015, 14, 1492–1509 RSC; (b) M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC; (c) A. Srivatsan, J. R. Missert, S. K. Upadhyay and R. K. Pandey, J. Porphyrins Phthalocyanines, 2015, 19, 109–134 CrossRef CAS.
- (a) J. J. Shi, L. Wang, J. Gao, Y. Liu, J. Zhang, R. Ma, R. Y. Liu and Z. Z. Zhang, Biomaterials, 2014, 35, 5771–5784 CrossRef CAS PubMed; (b) P. Meisel and T. Kocher, J. Photochem. Photobiol., B, 2005, 79, 159–170 CrossRef CAS PubMed; (c) S. Yano, S. Hirohara, M. Obata, Y. Hagiya, S.-I. Ogura, A. Ikeda, H. Kataoka, M. Tanaka and T. Joh, J. Photochem. Photobiol., C, 2011, 12, 46–67 CrossRef CAS PubMed.
- (a) C. A. Robertson, D. H. Evans and H. Abrahamse, J. Photochem. Photobiol., B, 2009, 96, 1–8 CrossRef CAS PubMed; (b) A. Juzeniene, Q. Peng and J. Moan, Photochem. Photobiol. Sci., 2007, 6, 1234–1245 RSC; (c) S. Bosi, T. D. Ros, G. Spalluto and M. Prato, Eur. J. Med. Chem., 2003, 8, 913–923 CrossRef PubMed.
- (a) M. D. Tzirakis and M. Orfanopoulos, Chem. Rev., 2013, 113, 5262 CrossRef CAS PubMed; (b) E. Nakamura and H. Isobe, Acc. Chem. Res., 2003, 36, 807–815 CrossRef CAS PubMed.
- P. Mroz, G. P. Tegos, H. Gali, T. Wharton, T. Sarna and M. R. Hamblin, Photochem. Photobiol. Sci., 2007, 6, 1139–1149 CAS.
- (a) A. Ikeda, Y. Doi, M. Hashizume, J. Kikuchi and T. Konishi, J. Am. Chem. Soc., 2007, 129, 4140–4141 CrossRef CAS PubMed; (b) Y. Doi, A. Ikeda, M. Akiyama, M. Nagano, T. Shigematsu, T. Ogawa, T. Takeya and T. Nagasaki, Chem. – Eur. J., 2008, 14, 8892–8897 CrossRef CAS PubMed; (c) A. Ikeda, K. Kiguchi, T. Shigematsu, K. Nobusawa, J. Kikuchi and M. Akiyama, Chem. Commun., 2011, 47, 12095–12097 RSC.
- (a) M. Guan, T. Qin, J. Ge, M. Zhen, W. Xu, D. Chen, S. Li, C. Wang, H. Su and C. Shu, J. Mater. Chem. B, 2015, 3, 776–783 RSC; (b) Y. Yang, M. Yu, H. Song, Y. Wang and C. Yu, Nanoscale, 2015, 7, 11894–11898 RSC; (c) W. Zhang, X. Gong, C. Liu, Y. Piao, Y. Sun and G. Diao, J. Mater. Chem. B, 2014, 2, 5107–5115 RSC.
- (a) Y. M. Chabre and R. Roy, Chem. Soc. Rev., 2013, 42, 4657–4708 RSC; (b) J.-F. Nierengarten, J. Iehl, V. Oerthel, M. Holler, B. M. Illescas, A. Muñoz, N. Martín, J. Rojo, M. Sanchez-Navarro, S. Cecioni, S. Vidal, K. Buffet, M. Durka and S. P. Vincent, Chem. Commun., 2010, 46, 3860–3862 RSC; (c) R. Rísquez-Cuadro, J. M. García Fernández, J.-F. Nirengarten and C. O. Mellet, Chem. – Eur. J., 2013, 19, 16791–16803 CrossRef PubMed; (d) I. Nierengarten and J.-F. Nierengarten, Chem. – Asian J., 2014, 9, 1436–1444 CrossRef CAS PubMed.
- (a) A. Vasella, P. Uhlmann, C. A. A. Waldraff, F. Diederich and C. Thilgen, Angew. Chem., Int. Ed. Engl., 1992, 31, 1388–1390 CrossRef PubMed; (b) R. F. Enes, A. C. Tomé, J. A. S. Cavaleiro, A. El-Agamey and D. J. McGarvey, Tetrahedron, 2005, 61, 11873–11881 CrossRef CAS PubMed; (c) W.-Q. Zhai, S.-P. Jiang, R.-F. Peng, B. Jin and G.-W. Wang, Org. Lett., 2015, 17, 1862–1865 CrossRef CAS PubMed.
- Y. Mikata, S. Takagi, M. Tanahashi, S. Ishii, M. Obata, Y. Miyamoto, K. Wakita, T. Nishisaka, T. Hirano, T. Ito, M. Hoshino, C. Ohtsuki, M. Tanihara and S. Yano, Bioorg. Med. Chem. Lett., 2003, 13, 3289–3292 CrossRef CAS.
- E. Otake, S. Sakuma, K. Torii, A. Maeda, H. Ohi, S. Yano and A. Morita, Photochem. Photobiol., 2010, 86, 1356–1363 CrossRef CAS PubMed.
- T. Tada, Y. Ishida and K. Saigo, J. Org. Chem., 2006, 71, 1633–1639 CrossRef CAS PubMed.
- J. Petrig, R. Schibli, C. Dumas, R. Alberto and P. A. Schubiger, Chem. – Eur. J., 2001, 7, 1868–1873 CrossRef CAS.
- Y. Chen, K. Ohkubo, M. Zhang, E. Wenbo, W. Liu, S. K. Pandey, M. Ciesielski, H. Baumann, S. Fukuzumi, K. M. Kadish, R. Fenstermaker, A. Oseroff and R. K. Pandey, Photochem. Photobiol. Sci., 2007, 6, 1257–1267 CAS.
- K. Ohkubo, N. Kohno, Y. Yamada and S. Fukuzumi, Chem. Commun., 2015, 51, 8082–8085 RSC.
- K. Ohkubo, J. Shao, Z. Ou, K. M. Kadish, G. Li, R. K. Pandey, M. Fujitsuka, O. Ito, H. Imahori and S. Fukuzumi, Angew. Chem., Int. Ed., 2004, 43, 853–856 CrossRef CAS PubMed.
- S. Fukuzumi, H. Mori, T. Suenobu, H. Imahori, X. Gao and K. M. Kadish, J. Phys. Chem. A, 2000, 104, 10688–10694 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods, Fundamental and Applications, John Wiley & Sons, New York, 2001, ch. 10, pp. 368–416 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and Fig. S1–S9, Table S1. See DOI: 10.1039/c5cc07353g |
| This journal is © The Royal Society of Chemistry 2015 |