Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oxidative addition of carbon–fluorine and carbon–oxygen bonds to Al(I)†
Mark R.
Crimmin
*,
Michael J.
Butler
and
Andrew J. P.
White
Department of Chemistry, Imperial College London, South Kensington, London SW7 2AZ, UK. E-mail: m.crimmin@imperial.ac.uk; Tel: +44 (0)2075942846
First published on 14th September 2015
Abstract
Addition of fluoroarenes, fluoroalkanes or benzofuran to [{(2,6-iPr2C6H3NCMe)2CH}Al] results in facile oxidative addition of either a C–F or C–O bond to the Al(I) centre.
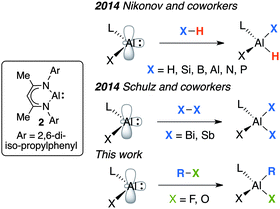
The addition of small molecules to ‘heavy carbenes’ of group 14 has led to the discovery of some remarkable reactivity.1 For example, ligand stabilised silylene, germylene and stannylene complexes react non-reversibly with fluorinated arenes through either carbon–fluorine or carbon–hydrogen bond functionalisation.2 In related studies of group 13 analogues, an isolable monomeric Al(I) complex has been reported to undergo oxidative addition of substrates containing H–H, O–H, N–H, P–H, acidic C–H, H–Si, H–B, H–Al, Bi–Bi and Sb–Sb bonds.3,4
For our part, we have shown that in the presence of catalytic quantities of [Cp2ZrCl2] or [Cp*RhCl(μ-Cl)]2, the aluminium dihydride 1 can be applied as a stoichiometric reagent for carbon–fluorine and carbon–oxygen bond cleavage in fluorocarbons and benzofuran (Fig. 1).5,6 In the case of fluorocarbons two competing pathways are observed: hydrodefluorination and C–F alumination.5
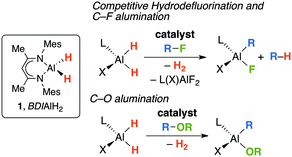 | ||
| Fig. 1 Transition metal catalysed R–F and R–OR bond functionalisation with the aluminium dihydride 1. | ||
One possible mechanism for R–X (X = F, OR) bond cleavage is through a transition-metal catalysed dehydrogenation of 1 to form an Al(I) intermediate.7 This low-valent intermediate could then effect carbon–fluorine or carbon–oxygen bond cleavage by oxidative addition. In the case of the fluoroarenes a competitive hydride transfer pathway could explain the hydrodefluorination products. While this hypothesis is just one of a number of possible mechanisms for R–X bond activation, two points lend weight to a hypothetical Al(III) → Al(I) transformation under catalytic conditions. (i) A recent detailed analysis of the electronic structure of 2 (Fig. 2) and the analogous Al(III) dihydride has exposed striking similarities between the charge density at aluminium in these complexes as evidenced by a combination of polarized aluminium K-edge X-ray absorption near edge structure (XANES) spectroscopy and calculations.8 (ii) While exploring the coordination chemistry of 1 we,9 and others,10 have shown that on-metal dehydrogenation is possible. Despite the rich bond activation chemistry of carbene analogues of the main group, the addition of R–X bonds to Al(I) has limited precedent.11 In this communication, we show that oxidative addition of C–F and C–O bonds to Al(I) is facile (Fig. 2). The findings mean that we cannot, as yet, discount the involvement of low-valent aluminium intermediates in the previous catalytic studies (Fig. 1).
The reaction of 2 with an excess of hexafluorobenzene or pentafluorobenzene in C6D6 proceeded rapidly at room temperature. Upon addition of the fluoroarene to a solution of 2 an instant colour change was observed from red/orange to a pale yellow. Following reactions by 1H and 19F NMR spectroscopy revealed the clean formation of 3a–b derived from the oxidative addition of an sp2 C–F bond to 2 (Scheme 1).
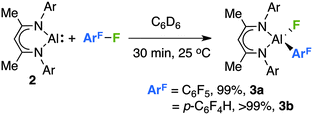 | ||
| Scheme 1 The reaction of 2 with fluoroarenes, yields are recorded by 1H NMR by comparison to ferrocene as an internal standard. | ||
No additional species were observed in solution and no evidence for competitive hydrodefluorination was obtained. For pentafluorobenzene, C–F bond cleavage occurs para to the existing carbon–hydrogen bond to give a product containing a 2,3,5,6-tetrafluoroarene motif. The regiochemistry in 3b was established through comparison of 19F NMR data with our previous findings,5 and 3b demonstrates 19F resonances at δ = −120.5 (m, ortho), −139.8 (m, meta) and −168 (broad s, Al–F) ppm. It is worth noting that the silylene analogue of 2 reacts with pentafluorobenzene by oxidative addition of a C–H bond.2a While reactions of 2 with 1,2,3-trifluorobenzene or fluorobenzene at 25–80 °C gave evidence for the slow formation of new aluminium fluoride species over 1–2 weeks, neither reaction cleanly produced analogues of 3a/b.
The scope of this transformation is not limited to the sp2 C–F bonds of fluoroarenes. Addition of 1-fluorohexane or fluorocyclohexane to 2 in C6D6 yielded the corresponding aluminium alkyls 3c–d through cleavage of the sp3 C–F bond (Scheme 2). In these instances, in addition to the expected aluminium fluoride resonances in 19F NMR spectra (3c, −161.8 ppm; 3d, −157.7 ppm) diagnostic aluminium alkyl resonances were observed between −0.2 and +0.3 ppm in 1H NMR spectra.
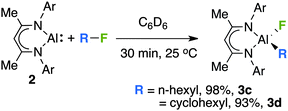 | ||
| Scheme 2 The reaction of 2 with fluoroalkanes, yields are recorded by 1H NMR by comparison to ferrocene as an internal standard. | ||
Although 2 did not react with benzofuran at 25 °C in C6D6, upon heating to 80 °C slow conversion to 4 was observed over a period of 24 h (Scheme 3). Diagnostic resonances of the aluminium vinyl unit of the product were observed at δ = 5.99 (d, 3JH–H = 16.0 Hz) and 7.44 (d, 3JH–H = 16.0 Hz) ppm. In contrast to the zirconocene dichloride catalysed reaction of 1 with benzofuran, no support for the formation of a saturated analogue of 4 was obtained.5 The reaction is selective for the formation of a 6-membered metallocycle containing a Z-alkenyl unit from oxidative addition of the sp2 C–O bond of benzofuran to Al(I).
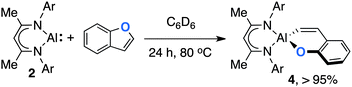 | ||
| Scheme 3 The reaction of 2 with benzofuran, yield is recorded by 1H NMR by comparison to ferrocene as an internal standard. | ||
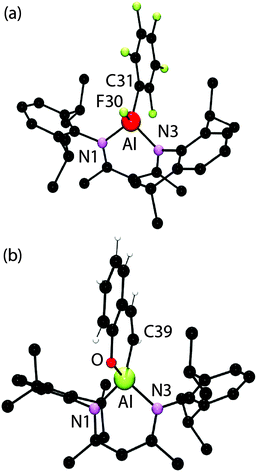
The organometallic products of these reactions have been characterised by multinuclear NMR spectroscopy and in the case of 3a and 4 by single crystal X-ray diffraction (Fig. 3). The aluminium–carbon and aluminium–fluorine bond lengths in the three independent complexes in 3a take values of 1.9916(19)–2.0020(18) and 1.6582(11)–1.6619(11) Å respectively. These data are consistent with those found in [BDIAl(C6F5)2] (1.9946(15) and 2.0198(15) Å) and [BDIAl(F)2] (1.6637(8) and 1.6647(8) Å).5 The crystal structure of an analogue of 4 has been previously reported.6
In summary, we have reported the facile oxidative addition of R–X (X = F, OR) bonds in fluoroarenes, fluoroalkanes and benzofuran to the Al(I) complex 2. From a mechanistic perspective, these data highlight the possibility that an in situ transition metal catalysed Al(III) → Al(I) transformation may be operating in our previously reported bond functionalisation chemistry employing the Al(III) dihydride 1.5,6 From a more pragmatic point of view, the reactions result in the generation of a new carbon–aluminium bond from ‘inert’ carbon–fluorine or carbon–oxygen bonds. This approach represents a method for directly generating reactive intermediates from non-reactive chemical feedstocks, that uses the most abundant metal in the earth's crust without the need for expensive or toxic transition metal catalysts.12,13
We are grateful to the Royal Society (MRC) and EPSRC (EP/L011514/1) for funding. Olga Ekkert is thanked for helpful discussions.
Notes and references
- (a) M. Haff, T. A. Schmedake and R. West, Acc. Chem. Res., 2000, 33, 704 CrossRef PubMed; (b) S. Yao, Y. Xiong and M. Driess, Organometallics, 2011, 30, 1748 CrossRef CAS.
- (a) A. Jana, P. P. Samuel, G. Tavcar, H. W. Roesky and C. Schulzke, J. Am. Chem. Soc., 2010, 132, 10164 CrossRef CAS PubMed; (b) R. Azhakar, H. W. Roesky, H. Wolf and D. Stalke, Chem. Commun., 2013, 49, 1841 RSC; (c) P. P. Samuel, A. P. Singh, S. P. Sarish, J. Matussek, I. Objartel, H. W. Roesky and D. Stalke, Inorg. Chem., 2013, 52, 1544 CrossRef CAS PubMed.
- T. Chu, I. Korobkov and G. I. Nikonov, J. Am. Chem. Soc., 2014, 136, 9195 CrossRef CAS PubMed.
- C. Ganesamoorthy, D. Bläser, C. Wölper and S. Schulz, Angew. Chem., Int. Ed., 2014, 53, 11587 CrossRef CAS PubMed.
- (a) S. Yow, S. J. Gates, A. J. P. White and M. R. Crimmin, Angew. Chem., Int. Ed., 2012, 51, 12559 CrossRef CAS PubMed; (b) O. Ekkert, S. D. A. Strudley, A. Rozenfeld, A. J. P. White and M. R. Crimmin, Organometallics, 2014, 33, 7027 CrossRef CAS.
- S. Yow, A. E. Nako, L. Neveu, A. J. P. White and M. R. Crimmin, Organometallics, 2013, 32, 5260 CrossRef CAS.
- C. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, H. Hao and F. Cimpoesu, Angew. Chem., Int. Ed., 2000, 39, 4274 CrossRef CAS.
- A. B. Altman, C. D. Pemmaraju, C. Camp, J. Arnold, S. G. Minasian, D. Prendergast, D. K. Shuh and T. Tyliszczak, J. Am. Chem. Soc., 2015, 137, 10304 CrossRef CAS PubMed.
- O. Ekkert, A. J. P. White, H. Toms and M. R. Crimmin, Chem. Sci., 2015, 6, 5617 RSC.
- (a) I. M. Riddlestone, S. Edmonds, P. A. Kaufman, J. Urbano, J. I. Bates, M. J. Kelly, A. L. Thompson, R. Taylor and S. Aldridge, J. Am. Chem. Soc., 2012, 134, 2551 CrossRef CAS PubMed; (b) I. M. Riddlestone, J. Urbano, N. Phillips, M. J. Kelly, D. Vidovic, J. I. Bates, R. Taylor and S. Aldridge, Dalton Trans., 2013, 42, 249 RSC; (c) J. A. B. Abdalla, I. M. Riddlestone, R. Tirfoin, N. Phillips, J. O. Bates and S. Aldridge, Chem. Commun., 2013, 49, 5547 RSC; (d) J. A. B. Abdalla, I. M. Riddlestone, J. Turner, P. A. Kaufman, R. Tirfoin, N. Phillips and S. Aldridge, Chem. – Eur. J., 2014, 20, 17624 CrossRef CAS PubMed; (e) J. Turner, J. A. B. Abdalla, J. I. Bates, R. Tirfoin, M. J. Kelly, N. Phillips and S. Aldridge, Chem. Sci., 2013, 4, 4245 RSC.
- For an example of a C–O bond cleavage product formed directly from the reduction of an Al(III) precursor in THF see: C. Schnitter, H. W. Roesky, C. Röpken, R. Herbst-Irmer, H.-G. Schmidt and M. Noltemeyer, Angew. Chem., Int. Ed., 1998, 37, 1952 CrossRef CAS.
- For examples of C–F borylation or silylation see: (a) Y. Ishii, N. Chatani, S. Yorimitsu and S. Murai, Chem. Lett., 1998, 157 CrossRef CAS; (b) T. Braun, F. Wehmeier and K. Altenhöner, Angew. Chem., Int. Ed., 2007, 46, 5321 CrossRef CAS PubMed; (c) T. Braun, M. A. Salomon, K. Altenhöner, M. Teltewskoi and S. Hinze, Angew. Chem., Int. Ed., 2009, 48, 1818 CrossRef CAS PubMed; (d) M. Teltewskoi, J. A. Panetier, S. A. Macgregor and T. Braun, Angew. Chem., Int. Ed., 2010, 49, 3947 CrossRef CAS PubMed; (e) S. I. Kalläne, M. Teltewskoi, T. Braun and B. Braun, Organometallics, 2015, 34, 1156 CrossRef; (f) W.-H. Guo, Q.-Q. Min, J.-W. Gu and X. Zhang, Angew. Chem., Int. Ed., 2015, 54, 9075 CrossRef CAS PubMed.
- For examples of C–O borylation see: (a) H. Kinuta, M. Tobisu and N. Chatani, J. Am. Chem. Soc., 2015, 137, 1593 CrossRef CAS PubMed; (b) C. Zarate, R. Manzano and R. Martin, J. Am. Chem. Soc., 2015, 137, 6754 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Including experimental procedures, X-ray crystallography data and characterisation data. CCDC 1419650-1419651. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc07140b |
| This journal is © The Royal Society of Chemistry 2015 |