Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes†
Abstract
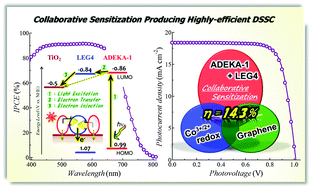
In dye-sensitized solar cells co-photosensitized with an alkoxysilyl-anchor dye ADEKA-1 and a carboxy-anchor organic dye LEG4, LEG4 was revealed to work collaboratively by enhancing the electron injection from the light-excited dyes to the TiO2 electrodes, and the cells exhibited a high conversion efficiency of over 14% under one sun illumination.
- This article is part of the themed collections: ChemComm contributions to the United Nations Sustainable Development Goals and ChemComm 60th Anniversary Historic Papers from Japan and South Korea

 Please wait while we load your content...
Please wait while we load your content...