Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
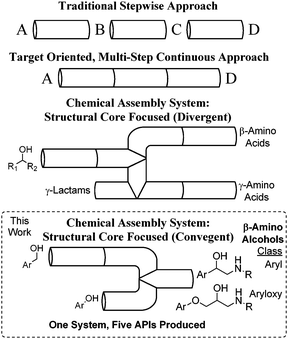
Continuous and convergent access to vicinyl amino alcohols†
Tomoya
Nobuta‡
a,
Guozhi
Xiao‡
a,
Diego
Ghislieri
a,
Kerry
Gilmore
 *a and
Peter H.
Seeberger
*ab
*a and
Peter H.
Seeberger
*ab
aMax Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, 14476 Potsdam, Germany. E-mail: kerry.gilmore@mpikg.mpg.de; peter.seeberger@mpikg.mpg.de
bInstitute of Chemistry and Biochemistry, Freie Universität Berlin, Arnimallee, 22, 14195 Berlin, Germany
First published on 24th August 2015
Abstract
Five active pharmaceutical ingredients (APIs) containing the vicinyl amino alcohol moiety were synthesized using a convergent chemical assembly system. The continuous system is composed of four flow reaction modules: biphasic oxidation, Corey–Chaykovsky epoxidation, phenol alkylation, and epoxide aminolysis. Judicious choice of reagents and module order allowed for two classes of β-amino alcohols, aryl and aryloxy, to be synthesized in good (27–69%) overall yields.
The chemical synthesis of active pharmaceutical ingredients (APIs) is generally accomplished via a linear batch approach where a single process produces a single compound. Flow chemistry is a conceptual advance that allows for greater control over reaction conditions1 and the ability to combine several sequential flow reactors to achieve multi-step processes.2–4 Chemical assembly systems (CAS)5 are based on telescoping sequential robust reaction units that are linked together in a non-iterative fashion. Multi-step syntheses of a wide range of compounds of similar6 or unique5 structural cores can be accessed in a divergent fashion (Fig. 1). Here, we report on the application of CAS to convergent syntheses, where two structural variations of a key epoxide intermediate can be trapped in an aminolysis reaction to produce two distinct classes of β-amino alcohols, including five APIs.
A variety of biologically active natural products, chiral auxiliaries, ligands, and APIs for the treatment of hypertension or as bronchodilators contain β-amino alcohols as an important structural core.7–9 Synthetic approaches to β-amino alcohols abound,10 including amino acid reduction,8a coupling reactions, α-functionalization,11 amino-hydroxylation, and epoxide ring opening.12 Two classes of pharmaceutically relevant compounds, aryl and aryloxy vicinyl amino alcohols, should be accessible via convergent epoxidations followed by a final ring opening with an amine (Fig. 2).
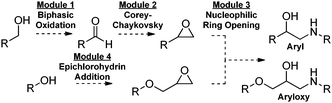 | ||
| Fig. 2 Convergent synthesis of aryl and aryloxy vicinyl amino alcohols via a concluding epoxide ring opening. | ||
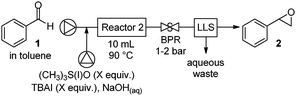
Aryl epoxides should be accessible via a one-to-two step oxidation/Corey–Chaykovsky process. Employing robust and selective biphasic alcohol-oxidation conditions we developed earlier,5 the first transformation is readily achieved and thus a continuous Corey–Chaykovsky epoxidation needed to be developed. Using benzaldehyde as a model system, a 0.5 M toluene solution was mixed with an aqueous solution of trimethylsulfonium iodide ((CH3)3S(I)O), sodium hydroxide (NaOH), and a substoichiometric amount of phase transfer catalyst (tetrabutylammonium iodide (TBAI)). The resulting biphasic solution was passed through a 10 mL reactor held at 90 °C at a pressure of 2 bar. Clean conversions to styrene oxide were observed with (CH3)3S(I)O and NaOH in excess (1.4–2 equiv., Table 1 entries 1–3) after 24 minutes. Shorter reaction times, temperature, and the absence of TBAI resulted in decreased conversion (entries 5–7).
| Entry | Me3SOIb | NaOHb | TBAIb | Residence time (min) | Conversion of 1 (%) |
|---|---|---|---|---|---|
| a Pump A: benzaldehyde (1) in toluene (0.5 M), flow rate: 0.083 mL min−1. Pump B: (CH3)3S(I)O (1.7 equiv., 0.21 M), NaOH (1.7 equiv.), TBAI (0.1 equiv.) in water, flow rate: 0.33 mL min−1, reactor size: 10 mL, pressure: 1–2 bar. b Equivalents with respect to benzaldehyde. c Reaction run at 60 °C. BPR: back pressure regulator, LLS: liquid/liquid separator. For full experimental details, see ESI. | |||||
| 1 | 1.4 | 1.4 | 0.1 | 24 | 95 |
| 2 | 1.7 | 1.7 | 0.1 | 24 | 97 |
| 3 | 2.0 | 2.0 | 0.1 | 24 | 96 |
| 4 | 1.7 | 1.7 | 0.1 | 40 | 96 |
| 5 | 1.7 | 1.7 | 0.1 | 10 | 88 |
| 6c | 1.7 | 1.7 | 0.1 | 24 | 85 |
| 7 | 1.7 | 1.7 | 0.0 | 24 | 79 |
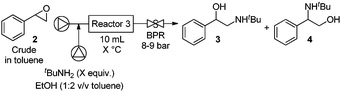
Using optimized conditions (97%, entry 2), we next turned to the amine ring-opening. While epoxide opening traditionally employed Lewis acids (LA), the elevated pressures and temperatures during microwave13 and flow12 transformations allow for clean reactions without LA catalysts. We aimed to combine epoxidation and ring opening by modifying established flow conditions.12 Thus, the crude toluene solution containing styrene oxide was mixed with tBuNH2 in toluene/ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). With three equivalents of amine, good conversion to the desired regioisomer was observed after 50 minutes at 150 °C (Table 2, entry 1). Increasing the amount of amine to seven equivalents resulted in the highest regioselectivity at essentially full conversion (entry 3). Decreasing both the temperature and time resulted in lower conversions (entries 4 and 5). No product resulting from the bis-alkylation of the amine was observed under these conditions.
1). With three equivalents of amine, good conversion to the desired regioisomer was observed after 50 minutes at 150 °C (Table 2, entry 1). Increasing the amount of amine to seven equivalents resulted in the highest regioselectivity at essentially full conversion (entry 3). Decreasing both the temperature and time resulted in lower conversions (entries 4 and 5). No product resulting from the bis-alkylation of the amine was observed under these conditions.
| Entry | t BuNH2 (equiv.) | Residence time (min) | Temp. (°C) | Ratiob3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
|---|---|---|---|---|
a Crude styrene oxide (2, 0.33 M in toluene), tBuNH2 in toluene/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), flow rates each 0.2 mL min−1. For full experimental details, see ESI.
b As determined by 1H NMR. BPR: back pressure regulator. 0.5), flow rates each 0.2 mL min−1. For full experimental details, see ESI.
b As determined by 1H NMR. BPR: back pressure regulator.
|
||||
| 1 | 3 | 50 | 150 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 2 | 5 | 50 | 150 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 3 | 7 | 50 | 150 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 4 | 5 | 50 | 130 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
| 5 | 5 | 20 | 150 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
Modules 2 and 3 were connected with the help of a modified Jensen liquid–liquid separator,14 allowing for the toluene phase exiting the epoxidation to be used directly in the ring opening reaction. Thus, after a total residence time of 74 minutes, benzaldehyde was transformed into 2-(tert-butylamino)-1-phenylethanol 3 in 57% yield following off-line crystallization. A range of haloarenes reacted with isopropyl or tert-butyl amine without intermediate purification using the modular set-up. Moderate to good yields of the desired regioisomer were observed following product crystallization (Fig. 3). Tulobuterol, a β2-adrenergic agonist used to treat asthma,15 was targeted. After passing 2-chloro benzyl alcohol through modules 1, 2, and 3 – with separators between modules 1/2 and 2/3 – the API was obtained in 27% yield (Fig. 3).
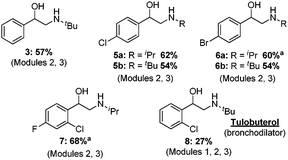 | ||
| Fig. 3 Aryl vicinyl amino alcohols produced by continuous synthesis. a Residence time of Corey–Chaykovsky reaction is 40 min. See ESI† for full experimental details. | ||
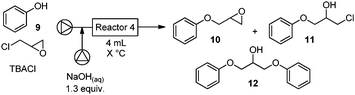
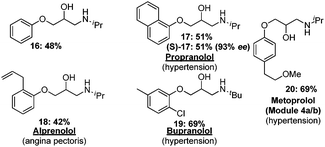
A number of hypertension medications such as propranolol, alprenolol, and metoprolol16 contain the aryloxy β-amino alcohol motif. These targets were selected to expand the scope of the modular flow synthesis of vicinyl amino alcohols. Typical phenol alkylations require a large excess of epichlorohydrin equivalents17 in the presence of an inorganic base.18 However, homogeneous solutions would facilitate the development of a flow process. A biphasic solution, obtained by mixing aqueous sodium hydroxide solution with a phenol in neat epichlorohydrin, should provide the desired aryloxy epoxide (Table 3).
| Entry | Epichlorohydrin (equiv.) | Temp. (°C) | TBACl (equiv.) | Residence time (min) | Yield (%) | ||
|---|---|---|---|---|---|---|---|
| 10 | 11 | 12 | |||||
| a Pump A: phenol, TBACl and epichlorohydrin, pump B: 1.95 M NaOH (1.3 equiv.), reactor size: 4 mL. For full experimental details, see ESI. | |||||||
| 1 | 3 | 35 | — | 60 | 56 | 24 | — |
| 2 | 3 | 35 | 0.1 | 60 | 68 | 15 | — |
| 3 | 3 | 45 | 0.1 | 60 | 86 | — | — |
| 4 | 3 | 45 | 0.1 | 46 | 80 | 8 | — |
| 5 | 2 | 45 | 0.1 | 60 | 85 | — | — |
| 6 | 1 | 45 | 0.1 | 60 | 65 | — | 21 |
The sodium hydroxide solution (1.95 M, 1.3 equiv.) is combined with phenol in three equivalents of neat epichlorohydrin via a T-mixer and passed through a reactor (1 h reaction time at 35 °C). Two major alkylation products (10, 11) were observed in roughly a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, with the desired epoxide 10 obtained in 56% (Table 3, entry 1). Addition of substoichiometric amounts of PTC resulted in an increased conversion of the intermediate halohydrin (11) into the desired product (entry 2). At a reaction temperature of 45 °C, 10 was obtained in 86% yield while no intermediate halohydrin was observed (entry 3). At higher temperatures, a diol was formed by sodium hydroxide mediated ring opening of the epoxide.19 Reducing the excess of epichlorohydrin to two equivalents (entry 5, final conditions) had no effect, while a further reduction of the reagent to one equivalent yielded sizable amounts of dimer 12 (entry 6). Shortening the residence time in the reactor to 46 minutes resulted in incomplete conversion (entry 4).
1 ratio, with the desired epoxide 10 obtained in 56% (Table 3, entry 1). Addition of substoichiometric amounts of PTC resulted in an increased conversion of the intermediate halohydrin (11) into the desired product (entry 2). At a reaction temperature of 45 °C, 10 was obtained in 86% yield while no intermediate halohydrin was observed (entry 3). At higher temperatures, a diol was formed by sodium hydroxide mediated ring opening of the epoxide.19 Reducing the excess of epichlorohydrin to two equivalents (entry 5, final conditions) had no effect, while a further reduction of the reagent to one equivalent yielded sizable amounts of dimer 12 (entry 6). Shortening the residence time in the reactor to 46 minutes resulted in incomplete conversion (entry 4).
Using this method, chiral aryloxy epoxides can be generated efficiently. When enantiopure epichlorohydrin is reacted using the conditions described above, the reaction proceeds with 93.7% ee (Fig. 4).
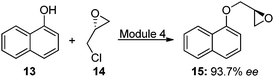 | ||
| Fig. 4 Synthesis of (S)-2-((naphthalen-1-yloxy)methyl)oxirane using enantiopure (R)-epichlorohydrin. | ||
Module 4 depends on the solubility of the intermediate aryloxide in the biphasic solution. In cases where a precipitate forms, as occurred during the synthesis of metoprolol 20, module 4 can split into a two-stage alkylation.20–22 Phenol was efficiently alkylated at 110 °C after 60 minutes when epichlorohydrin served as solvent (3.1 M, 2 equiv.). By mixing the resulting solution with aqueous NaOH at 45 °C, 84% of the desired epoxide could be obtained after only 30 minutes (Fig. 5).19
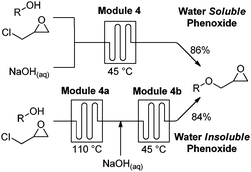 | ||
| Fig. 5 To accommodate water-soluble and insoluble phenoxides, two variants of module 4, were implemented. | ||
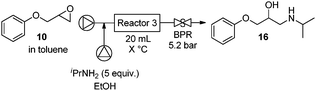
With a reliable process for the procurement of epoxide 10 in hand, the aminolysis module was connected (Table 2). Alkyl epoxides proved more active than aryl epoxides such that the ring opening proceeded at lower temperatures and shorter reaction times (Table 4). The epoxide solution was converted in just 20 min when mixed with five equivalents of isopropylamine in ethanol at 120 °C (entry 4). The reaction proceeds efficiently at 100 °C, but requires longer residence times (entry 2). Only one regioisomer and no amine dialkylation product were observed.
| Entry | T (°C) | Residence time (min) | Conversion (%) |
|---|---|---|---|
| a Pump A: epoxide in toluene, pump B: isopropylamine (5 equiv.) in EtOH, reactor size: 20 mL, BPR: 5.2 bar. For full experimental details, see ESI. BPR: back pressure regulator. | |||
| 1 | 80 | 30 | 78 |
| 2 | 100 | 30 | 100 |
| 3 | 100 | 20 | 85 |
| 4 | 120 | 20 | 100 |
Finally, modules 3 and 4 were combined to yield the final sequence. The biphasic solution exiting module 4 is mixed with toluene. The toluene phase is removed using a membrane-based separator14 and directly fed into module 3. Amino alcohol 16 was obtained in 48% yield from phenol, following off-line crystallization (Fig. 6). Several active pharmaceutical ingredients were prepared based on a judicious choice of starting materials. Racemic and chiral aryloxy β-amino alcohols can be prepared including propranolol (17, 51%),23 used to treat hypertension,24 alprenolol (18, 42%), an angina pectoris medication,25 and the hypertension drugs bupranolol (19, 69%),26 as well as metoprolol (20, 69%).
Described is the development of a convergent chemical assembly system consisting of four modules that reliably produces aryl and aryloxy β-amino alcohols in good yields. Modular flow units include biphasic oxidations,5 Corey–Chaykovsky epoxidations, aryloxy alkylations, and epoxide aminolysis.12 Aqueous in-line workup using liquid–liquid extractors resulted in continuous processes without intermediate purification. Starting from phenols or benzyl alcohols five active pharmaceutical ingredients for the treatment of hypertension, agina pectoris, and bronchiodilation were produced. Enantiopure aryloxy β-amino alcohols can be prepared by starting from R/S epichlorohydrin.
Notes and references
- (a) I. W. Davies and C. J. Welch, Science, 2009, 325, 701 CrossRef CAS PubMed; (b) J.-I. Yoshida, Y. Takahashi and A. Nagaki, Chem. Commun., 2013, 49, 9896 RSC; (c) L. Malet-Sanz and F. Susanne, J. Med. Chem., 2012, 55, 4062 CrossRef CAS PubMed.
- For recent selected reviews about flow chemistry, see: (a) V. Hessel, Chem. Eng. Technol., 2009, 32, 1655 CrossRef CAS PubMed; (b) D. Webb and T. F. Jamison, Chem. Sci., 2010, 1, 675 RSC; (c) R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502 CrossRef CAS PubMed; (d) N. G. Anderson, Org. Process Res. Dev., 2012, 16, 852 CrossRef CAS; (e) D. T. McQuade and P. H. Seeberger, J. Org. Chem., 2013, 78, 6384 CrossRef CAS PubMed; (f) K. S. Elvira, X. C. Solvas, R. C. R. Wootton and A. J. deMello, Nat. Chem., 2013, 5, 905 CrossRef CAS PubMed; (g) S. V. Ley, D. E. Fitzpatrick, R. J. Ingham and R. M. Myers, Angew. Chem., Int. Ed., 2015, 54, 3449 CrossRef CAS PubMed.
- J. C. Pastre, D. L. Browne and S. V. Ley, Chem. Soc. Rev., 2013, 42, 8849 RSC.
- For select examples, see: (a) M. D. Hopkin, I. R. Baxendale and S. V. Ley, Chem. Commun., 2010, 46, 2450 RSC; (b) A. R. Bogdan, S. L. Poe, D. C. Kubis, S. J. Broadwater and D. T. McQuade, Angew. Chem., 2009, 121, 8699 ( Angew. Chem., Int. Ed. , 2009 , 48 , 8547 ) CrossRef PubMed; (c) M. Viviano, T. N. Glasnov, B. Reichart, G. Tekautz and C. O. Kappe, Org. Process Res. Dev., 2011, 15, 858 CrossRef CAS; (d) D. R. Snead and T. F. Jamison, Chem. Sci., 2013, 4, 2822 RSC; (e) L. Kupracz and A. Kirschning, Adv. Synth. Catal., 2013, 355, 3375 CrossRef CAS PubMed; (f) M. D. Hopkin, I. R. Baxendale and S. V. Ley, Org. Biomol. Chem., 2013, 11, 1822 RSC; (g) T. Gustafsson, H. Sörensen and F. Pontén, Org. Process Res. Dev., 2012, 16, 925 CrossRef CAS; (h) F. Lévesque and P. H. Seeberger, Angew. Chem., 2012, 124, 1738 ( Angew. Chem., Int. Ed. , 2012 , 51 , 1706 ) CrossRef PubMed; (i) D. Kopetzki, F. Lévesque and P. H. Seeberger, Chem. – Eur. J., 2013, 19, 5450 CrossRef CAS PubMed; (j) D. R. Snead and T. F. Jamison, Angew. Chem., Int. Ed., 2015, 54, 983 CrossRef CAS PubMed; (k) C. A. Correia, K. Gilmore, D. T. McQuade and P. H. Seeberger, Angew. Chem., Int. Ed., 2015, 54, 4945 CrossRef CAS PubMed.
- D. Ghislieri, K. Gilmore and P. H. Seeberger, Angew. Chem., Int. Ed., 2014, 54, 678 Search PubMed.
- K. Gilmore, D. Kopetzki, J. W. Lee, Z. Horváth, D. T. McQuade, A. Seidel-Morgenstern and P. H. Seeberger, Chem. Commun., 2014, 50, 12652 RSC.
- (a) D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835 CrossRef CAS PubMed; (b) I. Gallou and C. H. Senanayake, Chem. Rev., 2006, 106, 2843 CrossRef CAS PubMed; (c) J. L. Vicario, D. Badia, L. Carrillo, E. Reyes and J. Etxebarria, Curr. Org. Chem., 2005, 9, 219 CAS; (d) F. Fache, E. Schulz, M. L. Tommasino and M. Lemaire, Chem. Rev., 2000, 100, 2159 CrossRef CAS PubMed.
- (a) D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835 CrossRef CAS PubMed; (b) E. J. Corey and F. Zhang, Angew. Chem., Int. Ed., 1999, 38, 1931 CrossRef CAS; (c) P. O'Brien, Angew. Chem., Int. Ed., 1999, 38, 326 CrossRef; (d) D. S. Bose and A. V. Narsaiah, Bioorg. Med. Chem., 2005, 3, 627 CrossRef PubMed.
- Amino alcohol drugs such as Metoprolol, Atenolol were among the Top 200 brand name drugs by total prescriptions in 2012, see: N. A. McGrath, M. Brichacek and J. T. Njardarson, J. Chem. Educ., 2010, 87, 1348 CrossRef CAS.
- S. C. Bermeier, Tetrahedron, 2000, 56, 561 Search PubMed.
- (a) S. Sohda, M. Fujimoto, T. Tamegai and N. Hirose, J. Med. Chem., 1979, 22, 279 CrossRef CAS; (b) E. Babad, N. I. Carruthers, R. S. Jaret and M. Steinman, Synthesis, 1988, 966 CrossRef CAS.
- M. W. Bedore, N. Zaborenko, K. F. Jensen and T. F. Jamison, Org. Process Res. Dev., 2010, 14, 432 CrossRef CAS.
- (a) H. Desai, B. R. D'Souza, D. Foether, B. F. Johnson and H. A. Lindsay, Synthesis, 2007, 902 CAS; (b) A. Robin, F. Brown, N. Bahamontes-Rosa, B. Wu, E. Beitz, J. F. J. Kun and S. L. Flitsch, J. Med. Chem., 2007, 50, 4243 CrossRef CAS PubMed; (c) P. Lidström, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225 CrossRef.
- For details of the modification, see ESI.† For the original description of the membrane-based separator, see J. G. Kralj, H. R. Sahoo and K. F Jensen, Lab Chip, 2007, 7, 256 RSC.
- T. Ichikawa and H. Sugiura, Res. Rep. Transdermal Drug Delivery, 2013, 2, 9 CrossRef.
- M. Sittig, Pharmaceutical manufacturing encyclopedia, Noyes publications, 2nd edn, 1988, vol. 1 Search PubMed.
- Typical conditions utilize epichlorohydrin in as much as 20× excess, see: ref. 16, p. 110.
- S. Srivastava, K. Bhandari, G. Shankar, H. K. Singh and A. K. Saxena, Med. Chem. Res., 2004, 13, 631 CrossRef CAS.
- See ESI† for further details.
- (a) K. Kawamura, T. Ohta and G. Otani, Chem. Pharm. Bull., 1990, 38, 2092 CrossRef CAS; (b) K. Sakaguchi, Y. Shiomi, T. Kitamura, Y. Takehira, M. Koden, T. Kuratate and K. Nakagawa, Chem. Lett., 1991, 1109 CrossRef CAS.
- For the use of CsF as catalyst, see: K. Kitaori, Y. Furukawa, H. Yoshimoto and J. Otera, Tetrahedron, 1999, 55, 14381 CrossRef CAS.
- For the achiral synthesis of aryl glycidyl ethers, see: Z.-Z. Liu, H.-C. Chen, S.-L. Cao and R.-T. Li, Synth. Commun., 1994, 24, 833 CrossRef CAS PubMed.
- This yield compares favourably with a comparable two-step batch process from 4-(benzyloxy)naphthalen-1-ol, see: T. Kumamoto, N. Aoyama, S. Nakano, T. Ishikawa and S. Narimatsu, Tetrahedron: Asymmetry, 2001, 12, 791 CrossRef CAS.
- “Propranolol hydrochloride”, Monograph, The American Society of Health-System Pharmacists, retrieved Jan 1, 2015.
- J. B. Hickie, Med. J. Aust., 1970, 2, 268 CAS.
- M. R. Candelore, L. Deng, L. Tota, X.-M. Guan, A. Amend, Y. Liu, R. Newbold, M. A. Cascieri and A. E. Weber, J. Pharmacol. Exp. Ther., 1999, 290, 649 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterization data, full experimental procedures, copies of 1H and 13C NMR spectra of all new compounds. See DOI: 10.1039/c5cc06093a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |