 Open Access Article
Open Access ArticleThe construction of a two-dimensional supramolecular organic framework with parallelogram pores and stepwise fluorescence enhancement†
Shun-Qi
Xu
,
Xiang
Zhang
,
Cheng-Bin
Nie
,
Zhong-Fu
Pang
,
Xiao-Na
Xu
and
Xin
Zhao
*
Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: xzhao@mail.sioc.ac.cn
First published on 28th September 2015
Abstract
A novel single-layer two-dimensional (2D) supramolecular organic framework (SOF) with parallelogram pores has been assembled to turn on the fluorescence emission of a non-emissive building block, and the emission could be further enhanced by the aggregation of the as-prepared 2D monolayers.
Inspired by the discovery of graphene, in recent years two-dimensional (2D) materials have become one of the most attractive subjects in materials science.1 Although currently many efforts have been devoted to the development of effective methods to construct 2D structures, the fabrication of this type of material has still been a great challenge.2 Very recently we reported the construction of the first single-layer 2D supramolecular organic framework (SOF) in water through cucurbit[8]uril (CB[8])-based host–guest chemistry.3 Lately we demonstrated that such 2D structures could have a significant impact on the properties of soft materials through the fabrication of highly thermally stable hydrogels from 2D SOFs.4 Due to their structural similarity to graphene, artificial 2D materials are also expected to display some intriguing features. However, the exploration of such features has been quite rare to date, as a result of the limited access to 2D structures.
Since the pioneering work of Tang and co-workers,5 aggregation-induced emission (AIE) or aggregation-induced enhanced emission (AIEE) has drawn considerable attention in the past decade because of their important applications in the fabrication of optical materials.6 The mechanism for the generation of AIE or AIEE is generally accepted to be the restriction of intramolecular rotation (RIR) of luminophores when they exist in the aggregating state.7 On the basis of this mechanism, a variety of materials which exhibit AIE or AIEE phenomenon have been designed and fabricated,8 among which tetraphenylethene (TPE) is the most widely used scaffold. In the construction of AIE/AIEE-based materials, self-assembly has been demonstrated to be an efficient tool to realize RIR through non-covalent interactions.9 In this context, self-assembled 2D structures should have the very advantage to confine luminophores in 2D space and thus should have great potential to facilitate AIE or AIEE. However, to the best of our knowledge, such a phenomenon has never been observed in a real 2D structure before. In this communication, we report the construction of a TPE-based 2D SOF with parallelogram pores. AIE was observed when the non-emissive monomers co-assembled with CB[8] to form a single-layer 2D SOF in water (Scheme 1). Its fluorescence was further enhanced when the monolayers were pushed to aggregate. The stepwise emission enhancement demonstrates interesting AIE and AIEE phenomena originating from the unique 2D SOF structure.
 | ||
| Scheme 1 The chemical structures of compound 1 and CB[8] and cartoon representation for the formation of a 2D SOF and stepwise fluorescence enhancement. | ||
The TPE-based building block (1) was designed by incorporating four 4,4′-bipyridin-1-ium (BP) units on the periphery of a TPE skeleton. We and Liu et al. have previously proved that CB[8] could encapsulate two BPs in its cavity in a head-to-tail arrangement so that supramolecularly polymeric structures could be assembled from CB[8] and rationally designed monomers.3,10 Directed by the preorganized conformation of the building block and the host–guest interaction, it is expected that a 2D SOF with parallelogram pores could be assembled from 1 and CB[8]. The 1H NMR titration experiment was firstly performed to confirm the formation of polymeric structures. As shown in Fig. 1, the addition of CB[8] to a solution of 1 in D2O resulted in the decrease of intensity and downfield shifts (except H1 and H2) of its peaks. The addition of 2.0 equiv. of CB[8] caused the signals of 1 to disappear completely. This result suggested that high-molecular-weight polymeric species formed in solution. Job's plot was further generated using UV-vis spectroscopy. It displayed a maximum absorption change at 33.3%, indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry for 1 and CB[8] (Fig. S1, ESI†).
2 stoichiometry for 1 and CB[8] (Fig. S1, ESI†).
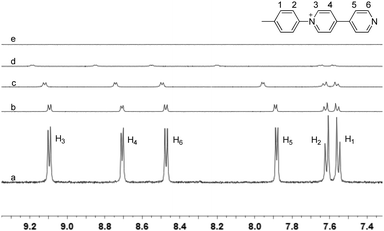 | ||
Fig. 1 Partial 1H NMR spectra of (a) 1, (b) 1 + CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2), (c) 1 + CB[8] (1 0.2), (c) 1 + CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), (d) 1 + CB[8] (1 0.5), (d) 1 + CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and (e) 1 + CB[8] (1 1), and (e) 1 + CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in D2O at 25 °C. The concentration of 1 was 0.25 mM. 2) in D2O at 25 °C. The concentration of 1 was 0.25 mM. | ||
The formation of large polymeric structures was further evidenced by the dynamic light scattering (DLS) experiment, which revealed that the hydrodynamic diameter (Dh) of the solution of the mixture of 1 and CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in water was 44.7 nm when the concentration of 1 was 0.25 mM (Fig. S2, ESI†). This value was 20 times larger than that of 1 alone in water at the same concentration. Concentration-dependence of Dh was also examined. It was found that the Dh value of the solution of the mixture of 1 and CB[8] (1
2) in water was 44.7 nm when the concentration of 1 was 0.25 mM (Fig. S2, ESI†). This value was 20 times larger than that of 1 alone in water at the same concentration. Concentration-dependence of Dh was also examined. It was found that the Dh value of the solution of the mixture of 1 and CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in water increased with the increasing concentration of the mixture (Fig. S2, ESI†), indicating a higher degree of polymerization at higher concentration, which is a typical feature of supramolecular polymers.
2) in water increased with the increasing concentration of the mixture (Fig. S2, ESI†), indicating a higher degree of polymerization at higher concentration, which is a typical feature of supramolecular polymers.
The morphology of the as-prepared material was then visualized using microscopes. The transmission electron microscopy (TEM) image revealed the presence of film-like structures with some corrugations, suggesting the formation of extremely thin 2D structures (Fig. 2a). The thickness of the films was measured by means of atomic force microscopy (AFM). AFM also revealed the formation of flat and uniform films with a height of about 1.7 nm, as indicated by cross-sectional analysis (Fig. 2b). This value agreed well with the diameter of rigid CB[8] (1.75 nm), confirming the formation of single-layer 2D SOFs.
In order to investigate the internal order of the as-formed 2D SOF which is a key criterion for a true 2D polymeric structure, the synchrotron small angle X-ray scattering (SAXS) experiment was carried out for the sample prepared by evaporating the solvent of an aqueous solution of 1 and CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). SAXS displayed a scattering peak corresponding to a d spacing of 2.11 nm (Fig. 2c). This value was well consistent with the theoretical pore width of the expected parallelogram pores (2.08 nm) of the SOF calculated using semi-empirical calculations, further confirming the formation of 2D SOF with periodic pore distribution.
2). SAXS displayed a scattering peak corresponding to a d spacing of 2.11 nm (Fig. 2c). This value was well consistent with the theoretical pore width of the expected parallelogram pores (2.08 nm) of the SOF calculated using semi-empirical calculations, further confirming the formation of 2D SOF with periodic pore distribution.
After the structure of the 2D SOF was corroborated, its properties were then investigated. Since in this 2D SOF the TPE units were confined in 2D space, we anticipated that the AIE effect might be generated in this unique architecture. As expected, monomer 1 was non-emissive in aqueous solution (Fig. 3a). However, upon addition of CB[8] into the solution of 1, fluorescence emission could be detected. The intensity of emission increased with the increasing amount of CB[8] and reached a maximum when 2.0 equiv. of CB[8] was added (Fig. 3a). This fluorescence emission turn-on phenomenon could be attributed to the formation of the single-layer 2D SOF, in which free rotation of the TPE units was restricted and thus the radiative channel was opened.
A typical feature of 2D architectures is that they are prone to aggregate to form layered structures, as a result of the large surface areas of sheet-like structures. Since the SOF is an ionic species, solvophobic interactions should arise between the SOF and the organic solvents in which SOF cannot be well solvated. We anticipated that the addition of organic solvents with low and medium polarity should push the as-prepared single-layer 2D SOF to aggregate in the solution phase to reduce their contacting areas with solvent molecules. In such a way the unfavourable solvophobic interactions could be alleviated. Water-miscible organic solvents such as acetone, acetonitrile, dioxane, and tetrahydrofuran (THF) were examined by introducing them into the solution of 2D SOF in water. Fluorescence emission of the resulting solutions was then recorded, which revealed that the addition of these organic solvents to the aqueous solution of SOF all resulted in enhancement of its fluorescence emission at different levels (Fig. S3, ESI†). Among the organic solvents tested, TFH did the best job, which should be attributed to its lowest polarity. Therefore, THF was selected as the poor solvent and more THF was further added. It was found that the fluorescence emission of the SOF remarkably increased upon the increased amount of THF (Fig. 3b). TEM investigation revealed that the 2D structure was still maintained even when 50% (v/v) THF was added (Fig. 4a). Moreover, the stacked layers could be observed from the TEM image, clearly indicating the aggregation of single-layer 2D SOFs. The DLS study confirmed again the aggregation of the monolayers in the presence of THF by demonstrating an increase of the hydrodynamic diameter of the species in the solution of 1 and CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with the increasing THF/water ratio of the binary solvent (Fig. 4b). Therefore, the enhancement of fluorescence emission of the 2D SOF after the addition of THF could be attributed to the aggregation of the single-layer SOF, which resulted in further inhibition of the rotation of TPE units in the stacked layers. In order to collect more evidence for this mechanism, a control experiment was performed by adding THF to aqueous solutions of 1 in the absence of CB[8]. It was found that the intensity of fluorescence emission of 1 could also be enhanced, but just to a small extent (Fig. S4, ESI†). This control experiment strongly suggested that it was the aggregation of a single-layer 2D SOF that further increased the fluorescence emission.
2) with the increasing THF/water ratio of the binary solvent (Fig. 4b). Therefore, the enhancement of fluorescence emission of the 2D SOF after the addition of THF could be attributed to the aggregation of the single-layer SOF, which resulted in further inhibition of the rotation of TPE units in the stacked layers. In order to collect more evidence for this mechanism, a control experiment was performed by adding THF to aqueous solutions of 1 in the absence of CB[8]. It was found that the intensity of fluorescence emission of 1 could also be enhanced, but just to a small extent (Fig. S4, ESI†). This control experiment strongly suggested that it was the aggregation of a single-layer 2D SOF that further increased the fluorescence emission.
In summary, a novel water soluble single-layer two-dimensional supramolecular organic framework with parallelogram pores has been constructed through the assembly of CB[8] and a TPE-based building block. Thanks to the unique features of the TPE skeleton and the rigid 2D polymeric structure, fluorescence emission of the non-emissive building block was turned on after it co-assembled with CB[8] to form a single-layer 2D SOF. By taking the advantage of the sheet-like structure of the 2D SOF, they could be further pushed to aggregate and thus the fluorescence emission could be further enhanced. This study demonstrates that interesting optical properties can be derived from intriguing 2D structures, which make such structures highly promising for the fabrication of novel 2D functional materials.
We thank the National Natural Science Foundation of China (No. 91127007, 21402228, and 21472225) for financial support and Shanghai Synchrotron Radiation Facility for providing BL16B1 beamline for collecting the synchrotron X-ray scattering data.
Notes and references
- R. Mas-Ballesté, C. Gómez-Navarro, J. Gómez-Herrero and F. Zamora, Nanoscale, 2011, 3, 20 RSC; J. Sakamoto, J. V. Heijst, O. Lukin and A. D. Schlüter, Angew. Chem., Int. Ed., 2009, 48, 1030 CrossRef CAS PubMed; S.-L. Cai, W.-G. Zhang, R. N. Zuckermann, Z.-T. Li, X. Zhao and Y. Liu, Adv. Mater., 2015 DOI:10.1002/adma. 201500124.
- T.-Y. Zhou, F. Lin, Z.-T. Li and X. Zhao, Macromolecules, 2013, 46, 7745 CrossRef CAS; K. Baek, G. Yun, Y. Kim, D. Kim, R. Hota, I. Hwang, D. Xu, Y. H. Ko, G. H. Gu, J. H. Suh, C. G. Park, B. J. Sung and K. Kim, J. Am. Chem. Soc., 2013, 135, 6523 CrossRef PubMed; K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451 CrossRef PubMed; P. Kissel, R. Erni, W. B. Schweizer, M. D. Rossell, B. T. King, T. Bauer, S. Gotzinger, A. D. Schlüter and J. Sakamoto, Nat. Chem., 2012, 4, 287 CrossRef PubMed; P. Kissel, D. J. Murray, W. J. Wulftange, V. J. Catalano and B. T. King, Nat. Chem., 2014, 6, 774 CrossRef PubMed; M. J. Kory, M. Wörle, T. Weber, P. Payamyar, S. W. van de Poll, J. Dshemuchadse, N. Trapp and A. D. Schlüter, Nat. Chem., 2014, 6, 779 CrossRef PubMed.
- K.-D. Zhang, J. Tian, D. Hanifi, Y. Zhang, A. C.-H. Sue, T.-Y. Zhou, L. Zhang, X. Zhao, Y. Liu and Z.-T. Li, J. Am. Chem. Soc., 2013, 135, 17913 CrossRef CAS PubMed , For example of SOF in the solid state, see: J. Lü, C. Perez-Krap, M. Suyetin, N. H. Alsmail, Y. Yan, S. Yang, W. Lewis, E. Bichoutskaia, C. C. Tang, A. J. Blake, R. Cao and M. Schröder, J. Am. Chem. Soc., 2014, 136, 12828 CrossRef PubMed.
- T.-Y. Zhou, Q.-Y. Qi, Q.-L. Zhao, J. Fu, Y. Liu, Z. Ma and X. Zhao, Polym. Chem., 2015, 6, 3018 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC; G. Liang, J. W. Y. Lam, W. Qin, J. Li, N. Xie and B. Z. Tang, Chem. Commun., 2014, 50, 1725 RSC; S. Zhang, J.-M. Yan, A.-J. Qin, J.-Z. Sun and B.-Z. Tang, Chin. Chem. Lett., 2013, 24, 668 CrossRef CAS PubMed; H. Shi, R. T. K. Kwok, J. Liu, B. Xing, B. Z. Tang and B. Liu, J. Am. Chem. Soc., 2012, 134, 17972 CrossRef PubMed; D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441 CrossRef PubMed.
- Y. Liu, S. M. Chen, J. W. Y. Lam, P. Lu, R. T. K. Kwok, F. Mahtab, H. S. Kwok and B. Z. Tang, Chem. Mater., 2011, 23, 2536 CrossRef CAS; Y. Liu, C. Deng, L. Tang, A. Qin, R. Hu, J. Z. Sun and B. Z. Tang, J. Am. Chem. Soc., 2011, 133, 660 CrossRef PubMed; Z. Chang, Y. Jiang, B. He, J. Chen, Z. Yang, P. Lu, H. S. Kwok, Z. Zhao, H. Qiu and B. Z. Tang, Chem. Commun., 2013, 49, 594 RSC; X. Du, J. Qi, Z. Zhang, D. Ma and Z. Y. Wang, Chem. Mater., 2012, 24, 2178 CrossRef.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429 CrossRef CAS PubMed.
- N. B. Shustova, B. D. McCarthy and M. Dincă, J. Am. Chem. Soc., 2011, 133, 20126 CrossRef CAS PubMed; Z. Wei, Z.-Y. Gu, R. K. Arvapally, Y.-P. Chen, R. N. McDougald, Jr., J. F. Ivy, A. A. Yakovenko, D. Feng, M. A. Omary and H.-C. Zhou, J. Am. Chem. Soc., 2014, 136, 8269 CrossRef PubMed; Y. Xu, L. Chen, Z. Guo, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2011, 133, 17622 CrossRef PubMed; Q. Chen, J.-X. Wang, F. Yang, D. Zhou, N. Bian, X.-J. Zhang, C.-G. Yan and B.-H. Han, J. Mater. Chem., 2011, 21, 13554 RSC; S. Ma, J. Zhang, J. Chen, L. Wang, B. Xu and W. Tian, Chin. J. Chem., 2013, 31, 1418 CrossRef PubMed; V. M. Suresh, S. Bonakala, S. Roy, S. Balasubramanian and T. K. Maji, J. Phys. Chem. C, 2014, 118, 24369 Search PubMed.
- G. Yu, G. Tang and F. Huang, J. Mater. Chem. C, 2014, 2, 6609 RSC; J. Wu, S. Sun, X. Feng, J. Shi, X.-Y. Hu and L. Wang, Chem. Commun., 2014, 50, 9122 RSC; J. Zhao, D. Yang, Y. Zhao, X.-J. Yang, Y.-Y. Wang and B. Wu, Angew. Chem., Int. Ed., 2014, 53, 1 CrossRef CAS PubMed; P. Wang, X. Yan and F. Huang, Chem. Commun., 2014, 50, 5017 RSC; X. Yao, X. Ma and H. Tian, J. Mater. Chem. C, 2014, 2, 5155 RSC; X. Wang, J. Hu, T. Liu, G. Zhang and S. Liu, J. Mater. Chem., 2012, 22, 8622 RSC; N. Song, D.-X. Chen, Y.-C. Qiu, X.-Y. Yang, B. Xu, W. Tian and Y.-W. Yang, Chem. Commun., 2014, 50, 8231 RSC; R. Chen, H. Jiang, H. Gu, Q. Zhou, J. Wu, D. Chen and J. Zhang, Chem. Commun., 2015, 51, 12220 RSC; M. V. R. Raju and H.-C. Lin, Org. Lett., 2014, 16, 5564 CrossRef PubMed.
- Z.-J. Zhang, H.-Y. Zhang, L. Chen and Y. Liu, J. Org. Chem., 2011, 76, 8270 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization, Job's plot, DLS profiles, and additional fluorescence spectra. See DOI: 10.1039/c5cc05875a |
| This journal is © The Royal Society of Chemistry 2015 |



