Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Converting 3D rigid metal–organic frameworks (MOFs) to 2D flexible networks via ligand exchange for enhanced CO2/N2 and CH4/N2 separation†
Yingdian
He
a,
Jin
Shang
*ab,
Qinfen
Gu
c,
Gang
Li
d,
Jiaye
Li
e,
Ranjeet
Singh
a,
Penny
Xiao
ab and
Paul A.
Webley
*ab
aDepartment of Chemical and Biomolecular Engineering, The University of Melbourne, Victoria 3010, Australia. E-mail: jin.shang@unimelb.edu.au; paul.webley@unimelb.edu.au; Tel: +61-3-90357873
bCooperative Research Center for Greenhouse Gas Technologies (CO2CRC), Melbourne, Australia
cAustralian Synchrotron, 800 Blackburn Rd, Clayton, Victoria 3168, Australia
dAustralian Centre for Energy, School of Mechanical and Chemical Engineering, The University of Western Australia, Crawley, WA 6009, Australia
eSchool of Chemistry, Monash University, Victoria 3800, Australia
First published on 31st July 2015
Abstract
We report a synthetic strategy for constructing a novel flexible MOF from a rigid parent structure by ligand exchange. This is the first reported study on introducing flexible heterogeneity into a rigid structure via substantial structural rearrangement. The daughter material exhibits enhanced gas separation selectivity compared with the parent.
Intensive studies on the development of technologies for adsorption-based gas separation have been driven worldwide by the increasing attention to the environment and the future energy demand. Adsorbents play an essential role in gas separation processes but conventional adsorbents (zeolites, activated carbon, silicon, etc.) are unable to meet the increasing demands placed by various industrial applications due to their limitations in structural diversity and flexibility. Novel adsorbents are required to improve gas separation performance and minimize the energy required for separation. Flexible metal–organic frameworks (MOFs) or porous coordination polymers (PCPs) are promising adsorbents for gas separation,1 since their pore aperture and pore volume can be tuned in response to external physical, mechanical, or chemical stimuli,2 thus achieving desired separation performance, such as high selectivity and capacity. However, the number of such flexible MOF candidates is relatively small compared with numerous rigid counterparts, because constructing phase-pure MOFs with flexible crystalline structure is difficult by typical de novo method.3 Moreover, it is very challenging to rationalize the design and control of flexible moieties in the intrinsic MOF structure by direct one-pot synthesis.
Recent alternative synthesis methods, termed solvent-mediated ligand exchange4 and solvent-assisted linker exchange (SALE),5 provide new options for the synthesis of novel MOFs with controllable flexibility and well-defined crystallinity. Ligand exchange is typically achieved by soaking parent MOF crystals in a concentrated ligand solution. Although the understanding of this synthesis method, such as the effect of solvent, modulator, temperature, and reaction kinetics, is still in its infancy, several pioneering studies have showed considerable advantages and interesting possibilities associated with those strategies.6 For example, solvent mediated ligand exchange was first investigated and successfully demonstrated to work for three metal–ligand coordination polymers [Ag(L)NO3]∞, (L = pyrazine, 3,4′-bipyridine, 4,4′-pipyridine, 4-(10-(pyridin-4-yl)anthracen-9-yl)pyridine).4 For another example, stepwise SALE has been recently reported to construct mesoporous MOFs by inserting longer pillars into the parent structure.7 It logically follows that ligand exchange could provide a versatile strategy for producing flexible MOFs as well: by replacing rigid ligands with flexible or semi-flexible ligands, it may be possible to rationally design and construct novel flexible MOFs.
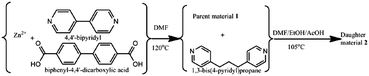
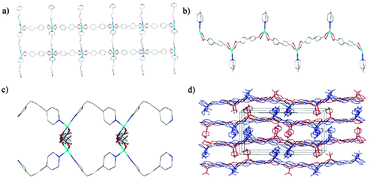
Herein, we report the conversion of a three-dimensional (3D) rigid framework MOF by ligand exchange into a novel two-dimensional (2D) flexible network whose pure-phase sample could not be obtained by direct synthesis in spite of many attempts in our lab. To the best of our knowledge, it is the first case of incorporating an exotic flexible-ligand into a rigid pillar-layered MOF structure via structural rearrangement during ligand exchange, creating structural flexibility in the daughter material. Interestingly, the adsorption properties of the daughter material suggest a superior gas separation performance to the parent material. The solvo-thermal reaction of Zn(CH3COO)2·2H2O, H2BPDC (BPDC = 4,4′-biphenyldicarboxylate) and BiPY (BiPY = 4,4′-bipyridine) produced colourless parent crystals 1.‡ Using 1 as precursor, the daughter material 2 was obtained by simply immersing 1 in a concentrated fresh 1,3-bis(4-pyridyl)propane (BPP) solution at 105 °C without stirring for four consecutive 3-day exchanges (Scheme 1). A detailed synthesis is provided in ESI.† Interestingly, a complete structural rearrangement occurred, with an entirely distinct pure-phase structure produced, as confirmed by synchrotron powder X-ray diffraction (PXRD) (Fig. 1). Specifically, the structure transferred from Orthorhombic (Pbcn) to Monoclinic (P21/c) with unit cell parameters provided in Table S1, ESI.† As shown in ESI,† Fig. S1, the crystal structure of the parent sample 1 consists of trinuclear Zn clusters which are connected to the carboxylate groups of six BPDC bridging linkers to form 2D double layers; and the secondary ligands BiPY, as pillars, support the 2D layers to generate a 3D framework. The structure of 1 is similar to those reported,8 in spite of the variance in reaction temperature and metal source in this work compared with the reported synthetic procedure, indicating the favoured coordination state in the system. In contrast, the daughter structure 2 exhibits a completely distinct crystallographic structure. Two oxygen atoms from the carboxylate group of two different BPDC are coordinated to one Zn centre, and the chains are connected by the Zn–N bond of the BPP whose N-to-N distance is approximately 8.5 Å. Thus, in this coordination system, BPP is expected to approach maximum stretching of the GG′ conformation which displays an N–N distance from 6.7 to 8.6 Å.9 The various conformations, due to the flexibility of the –(CH2)3– chain and the rotation of pyridyl rings, would be essential to the complete ligand exchange reaction. As seen from Fig. 2d, a buckled layer consists of two tangled networks (blue and red colours) in the ac plane; these buckled layers are then stacked up in the order of ababa along the b-axis. The daughter material exhibits a 2D flexible structure derived from both the unique topology and the bridging ligands, as further characterized below. To the best of our knowledge, it represents a rare example of 3D to 2D transformation during ligand replacement or desolvation, among the very large family of numerous MOFs.10
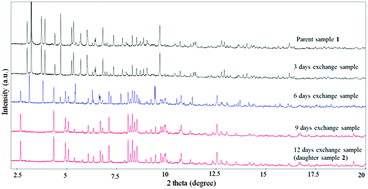 | ||
| Fig. 1 Synchrotron powder XRD of as-made parent 1 and samples with 3, 6, 9, and 12 days exchange time. | ||
Thermal gravimetric analysis (TGA) indicated 2 is thermally stable up to 316 °C in N2 atmosphere (ESI,† Fig. S2). The weight loss steps for the decomposition of BiPY of 1 and BPP of 2 occurred approximately at 372 and 318 °C, respectively. To better understand the mechanism of structure conversion from 1 to 2, we conducted synchrotron PXRD for samples collected at different reaction times: 3, 6, 9, and 12 days, to monitor the crystal phase transformation during the ligand exchange process. (Fig. 1) The parent crystal structure remained unchanged after 3 days exchange, and after 9 days, a new crystal phase of daughter material 2 formed. It is interesting that a heterogeneous product (approximately 40.1 wt% of 1 and 59.9 wt% of 2) was obtained for the 6 days sample, which may be indicative of a core–shell structure- this needs to be confirmed through more comprehensive microscopic characterisation. (ESI,† Plot S2) From the liquid 1H NMR analysis, the BiPY ligands in the parent material were completely replaced by BPP in the daughter material (ESI,† Fig. S3–S7). Scanning electron microscope (SEM) images show that the single crystals of parent sample 1 cracked into meso-crystals to form the structure of the daughter material 2, (ESI,† Fig. S8 and S9) rather than the single-crystal to single-crystal (SCSC) transformation which is usually observed during ligand exchange.11
Although several pioneering studies explored the chemical principles behind ligand exchange,12 the reaction mechanism in a certain coordination system is complicated and at present cannot be predicted a priori. In our case, acetic acid might act as a catalyst to promote the ligand exchange.13 The solvent-mediated reaction logically starts at the external surface of the parent crystals, and progresses into the inner structure. In addition, the stable secondary building unit (SBU) of the parent structure would experience disassembly, thus the underlying mechanism to undergo ligand exchange through the surface of the crystal is likely to be a dissolution/recrystallization process. As one of the family of pillared-paddlewheel MOFs, it is well known that the Zn–O bonds are significantly stronger than the Zn–N bonds,5,14 which enables the ligand exchange to occur by Zn–N bond cleavage and reforming involved in N-contained ligands rather than carboxylate ligands. Therefore, the topologies of the parent samples are sustained. However, the substantial structural arrangement observed in our work can be attributed to solvent-mediated ligand exchange (in contrast to a solid-state transformation), namely, the dissolution and recrystallization mechanism as described in the literature.4,15 The oxygen from deprotonated acetic acid may temporarily replace carboxylate oxygen and coordinate to the Zn ion, which promotes the disassembly of the parent metal cluster. Subsequently, the carboxylate ligands replace the oxygen of acetate acid, and BPP replaces BiPY completely to form a favourable topology via structural rearrangement.
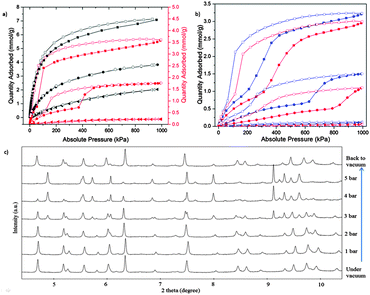
Importantly, the daughter material 2 exhibits guest molecule induced transformation from non-porous to porous structures. At 77 K and 1 bar, 1 adsorbs a substantial amount of N2 and H2: 12.0 and 9.4 mmol g−1, respectively (ESI,† Fig. S10). In striking contrast, the adsorption capacity of N2 and H2 on 2 were negligible under the same conditions, indicative of a non-porous crystal state. Hence, the conversion of the parent 3D structure to the daughter 2D structure would be beneficial to gas separation by excluding certain gases. Interestingly, CO2 and CH4 sorption on 2 up to 10 bar at 273, 298 and 313 K (Fig. 3a and b), show the typical isotherms for flexible MOFs – obvious adsorption steps with large hysteresis,16 which indicates structural flexibility and crystal phase transition upon gas sorption. To confirm the reversible structural transformation, we conducted in situ synchrotron PXRD depending on CO2 pressure. As shown in Fig. 3c, main peaks of the PXRD patterns of 2 shifted reversibly when the sample was under vacuum or various CO2 pressures, and the CO2 transition pressure is consistent with the step isotherm at 298 K (Fig. 3b). The adsorbate-induced structural transformations occurring in the case of CO2 and CH4 are attributed to strong interaction between gas molecules and the crystal, due to the relatively large quadrupole moment for CO2 of −1.43 × 10−39 C m2vs. N2 of −4.7 × 10−40 C m2, and polarizability for CH4 (25.9 × 10−25 cm−3vs. N2 of 17.4 × 10−25 cm−3). In other words, upon CO2 and CH4 adsorption, the non-porous desolvated 2 structure transformed to a porous network at the transition pressure, implying superior gas separation properties to the parent material 1. In contrast, the N2 adsorption amount is negligible up to 10 bar on 2 due to the weak adsorbate–adsorbent interactions, resulting in considerably enhanced CO2/N2 and CH4/N2 selectivity, compared with those for the parent material. Specifically, the daughter material exhibits ideal selectivity of CO2/N2 of 80 at 1 bar at 273 K based on pure component adsorption, compared with 8 for the parent sample. With regard to the CH4/N2 selectivity, the value increased from 1.86 to 8.2 at 10 bar at 273 K, which is comparable to the highest numbers in the open literature17 and holds promise for recovering CH4 from low-grade natural gas.
In conclusion, we report a novel route to convert a 3D rigid MOF structure to a 2D flexible network and the latter one shows reversible transformation from a non-porous to porous structure as induced by certain guest molecules, exhibiting a superior gas separation potential. We propose that the synthetic strategy can be potentially generalized and applied to other pillar-layered MOFs structures. This work provides more information on interpretation of substantial structural rearrangement via ligand exchange. Further investigations on the applicability of the synthetic methodology to other MOFs families are in progress.
Y.H. acknowledges the Chinese Scholarship Council (CSC). P.A.W. and J.S. acknowledge Australian Research Council (ARC) for providing the funding (DP2013000024). G.L. is a recipient of ARC DECRA (DE140101824). Part of work was undertaken on the PD beamline, Australian Synchrotron.
Notes and references
- (a) S. S. Nagarkar, A. V. Desai and S. K. Ghosh, Chem. – Asian J., 2014, 9, 2358–2376 CrossRef CAS PubMed; (b) J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed; (c) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- (a) R. Lyndon, K. Konstas, B. P. Ladewig, P. D. Southon, P. C. J. Kepert and M. R. Hill, Angew. Chem., Int. Ed., 2013, 3695–3698 CrossRef CAS PubMed; (b) N. Yanai, T. Uemura, M. Inoue, R. Matsuda, T. Fukushima, M. Tsujimoto, S. Isoda and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 4501–4504 CrossRef CAS PubMed.
- (a) C. R. Murdock, B. C. Hughes, Z. Lu and D. M. Jenkins, Coord. Chem. Rev., 2014, 258–259, 119–136 CrossRef CAS PubMed; (b) A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- X. Cui, A. N. Khlobystov, X. Chen, D. H. Marsh, A. J. Blake, W. Lewis, N. R. Champness, C. J. Roberts and M. Schröder, Chem. – Eur. J., 2009, 15, 8861–8873 CrossRef CAS PubMed.
- O. Karagiaridi, W. Bury, J. E. Mondloch, J. T. Hupp and O. K. Farha, Angew. Chem., Int. Ed. Engl., 2014, 53, 4530–4540 CrossRef CAS PubMed.
- P. Deria, J. E. Mondloch, O. Karagiaridi, W. Bury, J. T. Hupp and O. K. Farha, Chem. Soc. Rev., 2014, 43, 5896–5912 RSC.
- O. Karagiaridi, W. Bury, E. Tylianakis, A. A. Sarjeant, J. T. Hupp and O. K. Farha, Chem. Mater., 2013, 25, 3499–3503 CrossRef CAS.
- Q. Fang, X. Shi, G. Wu, G. Tian, G. Zhu, R. Wang and S. Qiu, J. Solid State Chem., 2003, 176, 1–4 CrossRef CAS.
- L. Carlucci, G. Ciani, D. M. Proserpio and S. Rizzato, CrystEngComm, 2002, 4, 121 RSC.
- (a) L.-J. Dong, C.-C. Zhao, X. Xu, Z.-Y. Du, Y.-R. Xie and J. Zhang, Cryst. Growth Des., 2012, 12, 2052–2058 CrossRef CAS; (b) Y. Kim, J. H. Song, W. R. Lee, W. J. Phang, K. S. Lim and C. S. Hong, Cryst. Growth Des., 2014, 14, 1933–1937 CrossRef CAS.
- T. Li, M. T. Kozlowski, E. A. Doud, M. N. Blakely and N. L. Rosi, J. Am. Chem. Soc., 2013, 135, 11688–11691 CrossRef CAS PubMed.
- (a) A. F. Gross, E. Sherman, S. L. Mahoney and J. J. Vajo, J. Phys. Chem. A, 2013, 117, 3771–3776 CrossRef CAS PubMed; (b) M. Kim, J. F. Cahill, Y. Su, K. A. Prather and S. M. Cohen, Chem. Sci., 2012, 3, 126–130 RSC; (c) O. Karagiaridi, M. B. Lalonde, W. Bury, A. A. Sarjeant, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 18790–18796 CrossRef CAS PubMed.
- M. Kim, J. F. Cahill, H. Fei, K. A. Prather and S. M. Cohen, J. Am. Chem. Soc., 2012, 134, 18082–18088 CrossRef CAS PubMed.
- S. Bureekaew, S. Amirjalayer and R. Schmid, J. Mater. Chem., 2012, 22, 10249 RSC.
- (a) A. N. Khlobystov, N. R. Champness, C. J. Roberts, S. J. Tendler, C. Thompson and M. Schröder, CrystEngComm, 2002, 4, 426–431 RSC; (b) F. Pan, J. Wu, H. Hou and Y. Fan, Cryst. Growth Des., 2010, 10, 3835–3837 CrossRef CAS.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed.
- X. Ren, T. Sun, J. Hu and S. Wang, RSC Adv., 2014, 4, 42326–42336 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, techniques, crystal data. CCDC 1404865. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc05867h |
| ‡ Crystal structure of parent sample 1 can refer to the previous reported structure (CCDC 192723). Crystal Structure of daughter material 2 (CCDC 1404865) was determined based on synchrotron powder XRD data, and detailed information is provided in ESI.† The formulae of the parent material 1 and daughter material 2 are [Zn3(C14H8O4)3(C10H8N2)] solvent and [Zn(C14H8O4)(C13H14N2)] solvent, respectively. |
| This journal is © The Royal Society of Chemistry 2015 |