Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A two-photon activatable amino acid linker for the induction of fluorescence†
Felix
Friedrich
a,
Kathrin
Klehs
b,
Manuela A. H.
Fichte
a,
Stephan
Junek
c,
Mike
Heilemann
*b and
Alexander
Heckel
*a
aGoethe University Frankfurt, Institute for Organic Chemistry and Chemical Biology, Max-von-Laue-Str. 9, 60438 Frankfurt, Germany. E-mail: heckel@uni-frankfurt.de
bGoethe University Frankfurt, Institute for Physical and Theoretical Chemistry, Max-von-Laue-Str. 9, 60438 Frankfurt, Germany. E-mail: heilemann@chemie.uni-frankfurt.de
cImaging facility, Max Planck Institute for Brain Research, Max-von-Laue-Straße 4, 60438 Frankfurt am Main, Germany
First published on 24th August 2015
Abstract
A new one- and two-photon activatable fluorophore based on ATTO565 was developed using a photolabile linker that simultaneously acts as a quencher. It is especially interesting for protein and peptide applications because it can be incorporated by standard peptide chemistry. The application of the new fluorogenic construct in super-resolution microscopy of antibody conjugates is shown.
New fluorescence imaging probes are of high interest for bioimaging at the subcellular level. For the control of fluorescence within biological or artificial systems in time and space, many different photoactivatable fluorophores were developed in the last few years.1 These caged fluorophores are weakly or non-fluorescent molecules. Irradiation with the appropriate wavelength releases the fluorescent dye. The use of light as an external trigger has the great advantage of being a non-invasive approach to control the time and area of the fluorescence if the wavelength is carefully chosen.
Photoactivatable fluorophores are also the key to single-molecule super-resolution techniques.2 In these techniques, the resolution limit of light microscopy is bypassed by a temporally confined detection of single fluorophores, the determination of their position and the reconstruction of an image from single-molecule coordinates.3,4 Organic fluorophores exhibit a high photon yield and typically show a better performance than fluorescent proteins; however, photoswitching often requires the addition of redox chemicals to the image buffer which are cytotoxic. In order to achieve the best results in single-molecule super-resolution imaging, it is thus desirable to develop caged organic fluorophores which exhibit a high contrast ratio between the fluorescent and the quenched state.
There are different strategies for the development of caged organic fluorophores. A simple but very effective strategy is to attach a photolabile group to the fluorophore at a position that interrupts the conjugated π-system and suppresses fluorescence. Since the first reports in this field (using fluorescein derivatives),5 the strategy was also applied to other chromophore classes like rhodamines,6 acridinone,7 rosamine,8 coumarins,9 carborhodamines10 and boron-dipyrromethane (BODIPY).11 Also, the efficiency and the properties of the photolabile groups were enhanced in different studies.12 In recent publications the use of 2-diazoketone as a very small caging group for fluorophores was presented. Irradiation of these molecules releases nitrogen and causes a rearrangement reaction resulting in the recovery of fluorescence. This strategy was applied to a variety of different fluorophores and their application in super-resolution microscopy was shown.13
Other approaches that generate light activatable fluorophores without the use of caging groups trigger, for instance, the photoinitiated oxidation of a ZnSalen from a quenched thioether to a fluorescent sulfoxide.14 Another example of a quenched cyanine dye used the photocleavage of a C–Br bond to restore the fluorescence. The fluorescence is quenched by bromides by the induction of intersystem crossing.15 A completely different strategy uses photoactivated click chemistry of a tetrazole with different dipolarophiles to form fluorescent cycloadducts. Irradiation of tetrazole induces the elimination of nitrogen. The resulting highly reactive nitrile imine can form fluorescent products with appropriate alkenes.16
The release of nitrogen was also used in the design of photoactivatable push–pull fluorophores.17 The electron donating amino group is masked as an azide moiety. Upon illumination, the aryl azide releases nitrogen and is transformed into an aryl amine, thus restoring fluorescence. The compatibility of these systems with super-resolution imaging was also demonstrated.18
An alternative way to trigger fluorescence with light is to attach a fluorophore and a quencher in close proximity. The photolabile groups act either as a linker between the fluorophore and the quencher19 or the cages force a structure in a special conformation. Upon irradiation the separation of the fluorophore and quencher takes place and fluorescence is restored. For instance, both of these techniques were used to follow mRNA distribution in cells with molecular beacons.20
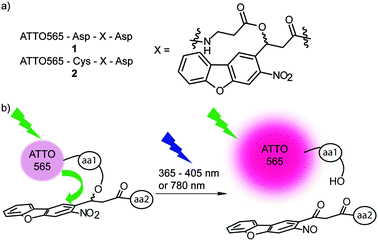
In this work we present a new strategy where the photolabile group is not directly attached to the fluorophore. Our newly synthesized linker molecule combines the properties of a quencher and a photolabile group (Fig. 1). This linker is especially interesting for protein and peptide applications because it can be incorporated into standard peptide coupling chemistry. We prepared small peptides containing the new linker and investigated the photochemical properties of the new constructs using one-photon excitation (shown here for 365–568 nm) and two-photon excitation (shown here for 780 nm). To the best of our knowledge the new linker is the first example of a photolabile quencher.
Furthermore, the new construct offers the possibility to be used as a strand break that can be activated with IR light. Strand breaks are prominent tools to control biomolecules such as oligonucleotides21 and peptides.22 Future applications in which the linker could trigger self-assembly processes23 or control the activity of enzymes and proteins with high spatial resolution24 is possible.
We observed in a previous study that the nitrodibenzofuran (NDBF) caging group possesses the ability to quench the fluorescence of the rhodamine fluorophore ATTO565 presumably by contact quenching.25 The NDBF group is known to be cleaved by UV light and also above 400 nm.26 NDBF possesses a good two-photon absorption cross-section, which allows the triggering of the photoreaction with IR light.27
The first step of the synthesis was adapted from an already published procedure.28 4-Fluoro-2-nitro-benzaldehyde was coupled with 2-iodophenol in an Ullmann reaction. The resulting aldehyde was protected as an acetal and the dibenzofuran core was generated by palladium-catalysed cross-coupling. After acidic deprotection, the aldehyde was transferred to an alcohol by the Reformatsky reaction and further coupled to Fmoc-β-alanine. Final deprotection of carboxylic acid resulted in the new linker, which can then be used in standard Fmoc peptide coupling chemistry (Scheme 1).
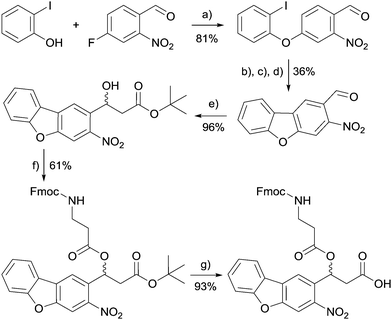 | ||
| Scheme 1 Synthesis scheme of the new linker molecule. (a) CuBr, K2CO3. (b) Ethyleneglycol, p-toluenesulfonic acid. (c) Cs2CO3, Pd(OAc)2. (d) HCl. (e) Rieke-Zn, tert-butyl chloroacetate. (f) Fmoc-β-alanine, EDC, DMAP. (g) TFA. For detailed procedures and analytical data see the ESI.† | ||
First, we checked if base-induced elimination is a problem in further applications of the new linker and confirmed that it is sufficiently stable in 20% (v/v) piperidin in DMF – a typical cleavage condition for Fmoc groups in the solid-phase peptide synthesis (ESI†).
To study our new linker it was embedded into two short peptides 1 and 2 (Fig. 1). Both contained aspartic acid residues for solubility purposes and an optional cysteine (peptide 2) for maleimide coupling. The N-terminus was labelled with an ATTO565-N-hydroxysuccinimide (NHS) active ester. Peptide 1 was soluble in small volumes of aqueous Tris and HEPES buffered saline (HBS) buffers (pH 7.5) to at least 2 mM. We observed less than 5% degradation of the linker over 170 h in the dark and at room temperature in HBS buffer.
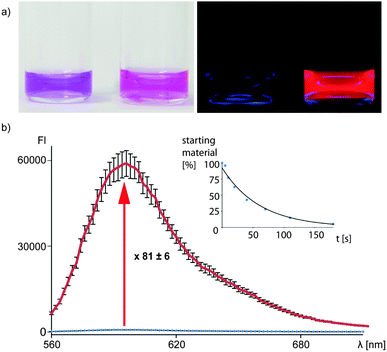
The uncaging quantum yield of peptide 1 was determined to be 0.13 at 365 nm using ferrioxalate actinometry.29 A significant fluorescence increase of this compound was observed upon uncaging (81 ± 6-fold increase in the absolute fluorescence, Fig. 2, 22-fold increase in fluorescence quantum yield, ESI†). This makes the new construct very interesting for bioimaging applications. The dye could also be photoactivated using a widefield microscope at 405 nm illumination and even at 568 nm (ESI†).
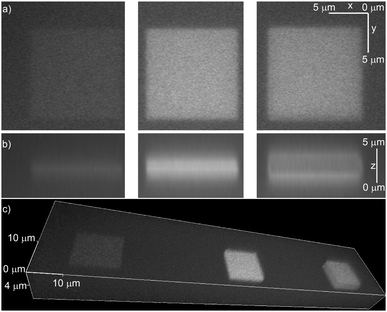
To prove that the NDBF group incorporated into the peptides is still two-photon active, molecule 2 was immobilized in a maleimide-containing hydrogel. The irradiation was performed using a laser-scanning microscope at 780 nm in a defined square region with increasing laser power (Fig. 3). Fig. 3b (middle) shows that indeed an excellent three-dimensional activation could be obtained by two-photon excitation. At high laser intensities bleaching of ATTO565 in the middle of the focal plane was observed (Fig. 3b, right).
At the correct settings two-photon irradiation offers the possibility to activate and bleach the fluorophore consecutively. Due to the activation with IR light, this procedure allows high tissue penetration depths with little phototoxicity.
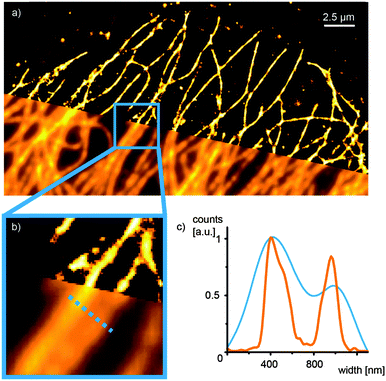
To make the new construct a valuable marker for biomolecules, peptide 2 was reacted with 6-maleimidohexanoic acid N-hydroxysuccinimide ester, a bifunctional linker. The resulting NHS ester was coupled to an antibody and used for staining of microtubules of U2OS cells via immunofluorescence. These filamentary structures are an ideal target to evaluate the suitability of a fluorophore for super-resolution microscopy following the concept of single-molecule localization.3
We demonstrated one-photon activation of the antibody conjugate with a short 405 nm laser pulse and continuous illumination at 568 nm (ESI,† Fig. S5). Furthermore, we illuminated the sample at 568 nm only and found a consecutive activation and bleaching or blinking of single peptide 2 molecule at appropriate laser intensities (ESI,† Fig. S6). We used standard localization algorithms to fit a 2D Gaussian function to the diffraction-limited intensity profile of each single emitter in order to determine their center of mass. Reconstruction of these localizations in a 2D histogram yielded an image with a significantly improved spatial resolution (Fig. 4) and an experimental localization uncertainty of 20.4 nm determined by nearest neighbor analysis.30 Notably, we thereby demonstrate single-molecule super-resolution imaging with synthetic fluorophores without the need for chemical additives in the imaging buffer.4
In summary, we developed the first photolabile quencher for ATTO565 with a light/dark contrast of 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It can be incorporated into peptides and acts both as a quencher and a strand break. The easy way to the functionalization of the linker with other molecules like hydrophilic amino acids results in a good solubility in aqueous buffers (pH 7.5). The linker shows very little degradation under these conditions. Irradiation can be performed with UV light, also above 400 nm, which is more favourable for living cells. Additionally, the construct is two-photon accessible that allows very high spatio-temporal resolution and high tissue penetration depths in the possible applications. The linker provides a new tool in the field of bioimaging. It reduces the number of modifications needed to generate a photoactivatable fluorophore quencher system from three (fluorophore, quencher and photolabile groups) to two (fluorophore and new linker molecule). In addition, the high contrast between the fluorescent and the dark state makes this probe ideally suited for single-molecule super-resolution microscopy. In the future, we will investigate the mechanism of the quenching effect and develop an application of the new linker to other fluorophores.
1. It can be incorporated into peptides and acts both as a quencher and a strand break. The easy way to the functionalization of the linker with other molecules like hydrophilic amino acids results in a good solubility in aqueous buffers (pH 7.5). The linker shows very little degradation under these conditions. Irradiation can be performed with UV light, also above 400 nm, which is more favourable for living cells. Additionally, the construct is two-photon accessible that allows very high spatio-temporal resolution and high tissue penetration depths in the possible applications. The linker provides a new tool in the field of bioimaging. It reduces the number of modifications needed to generate a photoactivatable fluorophore quencher system from three (fluorophore, quencher and photolabile groups) to two (fluorophore and new linker molecule). In addition, the high contrast between the fluorescent and the dark state makes this probe ideally suited for single-molecule super-resolution microscopy. In the future, we will investigate the mechanism of the quenching effect and develop an application of the new linker to other fluorophores.
FF and AH gratefully acknowledge the Konrad-Adenauer-Stiftung for a fellowship for FF. The authors thank Erin Schuman for generous access to the microscopes at the MPI for brain research. We are grateful to Sebastian Malkusch for providing the LAMA software. This work was funded by the Deutsche Forschungsgemeinschaft (EXC 115).
Notes and references
- For reviews see for example: (a) W. Li and G. Zheng, Photochem. Photobiol. Sci., 2012, 11, 460 RSC; (b) F. M. Raymo, Phys. Chem. Chem. Phys., 2013, 15, 14840 RSC; (c) D. Puliti, D. Warther, C. Orange, A. Specht and M. Goeldner, Bioorg. Med. Chem., 2011, 19, 1023 CrossRef CAS; (d) F. M. Raymo, J. Phys. Chem. Lett., 2012, 3, 2379 CrossRef CAS; (e) F. M. Raymo, ISRN Phys. Chem., 2012, 2012, 1 CrossRef.
- A. Fürstenberg and M. Heilemann, Phys. Chem. Chem. Phys., 2013, 15, 14919 RSC.
- (a) E. Betzig, G. H. Patterson, R. Sougrat, O. W. Lindwasser, S. Olenych, J. S. Bonifacino, M. W. Davidson, J. Lippincott-Schwartz and H. F. Hess, Science, 2006, 313, 1642 CrossRef CAS; (b) S. T. Hess, T. P. K. Girirajan and M. D. Mason, Biophys. J., 2006, 91, 4258 CrossRef CAS.
- (a) M. J. Rust, M. Bates and X. Zhuang, Nat. Methods, 2006, 3, 793 CrossRef CAS; (b) M. Heilemann, S. van de Linde, M. Schüttpelz, R. Kasper, B. Seefeldt, A. Mukherjee, P. Tinnefeld and M. Sauer, Angew. Chem., 2008, 120, 6266 ( Angew. Chem., Int. Ed. , 2008 , 47 , 6172 ) CrossRef.
- G. A. Krafft, W. R. Sutton and R. T. Cummings, J. Am. Chem. Soc., 1988, 110, 301 CrossRef CAS.
- (a) J. Ottl, D. Gabriel and G. Marriott, Bioconjugate Chem., 1998, 9, 143 CrossRef CAS; (b) K. R. Gee, E. S. Weinberg and D. J. Kozlowski, Bioorg. Med. Chem. Lett., 2001, 11, 2181 CrossRef CAS.
- D. Warther, F. Bolze, J. Léonard, S. Gug, A. Specht, D. Puliti, X.-H. Sun, P. Kessler, Y. Lutz, J.-L. Vonesch, B. Winsor, J.-F. Nicoudand and M. Goeldner, J. Am. Chem. Soc., 2010, 132, 2585 CrossRef CAS.
- W. Lin, L. Long, W. Tan, B. Chen and L. Yuan, Chem. – Eur. J., 2010, 16, 3914 CrossRef CAS.
- (a) Y. Zhao, Q. Zheng, K. Dakin, K. Xu, M. L. Martinez and W.-H. Li, J. Am. Chem. Soc., 2004, 126, 4653 CrossRef CAS; (b) C. Orange, A. Specht, D. Puliti, E. Sakr, T. Furuta, B. Winsor and M. Goeldner, Chem. Commun., 2008, 1217 RSC.
- J. B. Grimm, A. J. Sung, W. R. Legant, P. Hulamm, S. M. Matlosz and E. Betzigand L. D. Lavis, ACS Chem. Biol., 2013, 8, 1303 CrossRef CAS.
- S. S. Agasti, A. M. Laughney, R. H. Kohler and R. Weissleder, Chem. Commun., 2013, 49, 11050 RSC.
- (a) L. M. Wysocki, J. B. Grimm, A. N. Tkachuk, T. A. Brown, E. Betzig and L. D. Lavis, Angew. Chem., 2011, 123, 11402 ( Angew. Chem., Int. Ed. , 2011 , 50 , 11206 ) CrossRef; (b) E. E. Nekongo and V. V. Popik, J. Org. Chem., 2014, 79, 7665 CrossRef CAS; (c) K. Furukawa, H. Abe, S. Tsuneda and Y. Ito, Org. Biomol. Chem., 2010, 8, 2309 RSC; (d) T. Kobayashi, Y. Urano, M. Kamiya, T. Ueno, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 6696 CrossRef CAS.
- (a) V. N. Belov, C. A. Wurm, V. P. Boyarskiy, S. Jakobs and S. W. Hell, Angew. Chem., 2010, 122, 3598 ( Angew. Chem., Int. Ed. , 2010 , 49 , 3520 ) CrossRef; (b) V. N. Belov, G. Y. Mitronova, M. L. Bossi, V. P. Boyarskiy, E. Hebisch, C. Geisler, K. Kolmakov, C. A. Wurm, K. I. Willig and S. W. Hell, Chem. – Eur. J., 2014, 20, 13162 CrossRef CAS; (c) K. Kolmakov, C. Wurm, M. V. Sednev, M. L. Bossi, V. N. Belov and S. W. Hell, Photochem. Photobiol. Sci., 2012, 11, 522 RSC.
- J. Jing, J.-J. Chen, Y. Hai, J. Zhan, P. Xu and J.-L. Zhang, Chem. Sci., 2012, 3, 3315 RSC.
- Y. Hao, X.-Z. Dong and Y. Chen, New J. Chem., 2014, 38, 3468 RSC.
- (a) Z. Yu, L. Y. Ho and Q. Lin, J. Am. Chem. Soc., 2011, 133, 11912 CrossRef CAS; (b) P. An, Z. Yu and Q. Lin, Chem. Commun., 2013, 49, 9920 RSC; (c) Z. Yu, T. Y. Ohulchanskyy, P. An, P. N. Prasad and Q. Lin, J. Am. Chem. Soc., 2013, 135, 16766 CrossRef CAS; (d) P. An, Z. Yu and Q. Lin, Org. Lett., 2013, 15, 5496 CrossRef CAS.
- (a) S. J. Lord, N. R. Conley, H. D. Lee, R. Samuel, N. Liu, R. J. Twieg and W. E. Moerner, J. Am. Chem. Soc., 2008, 130, 9204 CrossRef CAS; (b) S. J. Lord, H. D. Lee, R. Samuel, R. Weber, N. Liu, N. R. Conley, M. A. Thompson, R. J. Twieg and W. E. Moerner, J. Phys. Chem. B, 2010, 114, 14157 CrossRef CAS.
- (a) H. D. Lee, S. J. Lord, S. Iwanaga, K. Zhan, H. Xie, J. C. Williams, H. Wang, G. R. Bowman, E. D. Goley, L. Shapiro, R. J. Twieg, J. Rao and W. E. Moerner, J. Am. Chem. Soc., 2010, 132, 15099 CrossRef CAS; (b) M. K. Lee, J. Williams, R. J. Twieg, J. Rao and W. E. Moerner, Chem. Sci., 2013, 4, 220 RSC.
- (a) H.-M. Lee, W. Xu and D. S. Lawrence, J. Am. Chem. Soc., 2011, 133, 2331 CrossRef CAS; (b) H.-M. Lee, M. A. Priestman and D. S. Lawrence, J. Am. Chem. Soc., 2010, 132, 1446 CrossRef CAS.
- (a) K. B. Joshi, A. Vlachos, V. Mikat, T. Deller and A. Heckel, Chem. Commun., 2012, 48, 2746 RSC; (b) J. S. Rinne, T. P. Kaminski, U. Kubitscheck and A. Heckel, Chem. Commun., 2013, 49, 5375 RSC.
- A. Dussy, C. Meyer, E. Quennet, T. A. Bickle, B. Giese and A. Marx, ChemBioChem, 2002, 3, 54 CrossRef CAS.
- F. Rohrbach, F. Schäfer, M. A. H. Fichte, F. Pfeiffer, J. Müller, B. Pötzsch, A. Heckel and G. Mayer, Angew. Chem., 2013, 125, 12129 ( Angew. Chem., Int. Ed. , 2013 , 52 , 11912 ) CrossRef.
- C. J. Bosques and B. Imperiali, J. Am. Chem. Soc., 2003, 125, 7530 CrossRef CAS.
- (a) H. Li, J.-M. Hah and D. S. Lawrence, J. Am. Chem. Soc., 2008, 130, 10474 CrossRef CAS; (b) N. Umezawa, Y. Noro, K. Ukai, N. Kato and T. Higuchi, ChemBioChem, 2011, 12, 1694 CrossRef CAS.
- V. Gatterdam, R. Ramadass, T. Stoess, M. A. H. Fichte, J. Wachtveitl, A. Heckel and R. Tampé, Angew. Chem., 2014, 126, 5787 ( Angew. Chem., Int. Ed. , 2014 , 53 , 5680 ) CrossRef.
- (a) F. Schäfer, K. B. Joshi, M. A. H. Fichte, T. Mack, J. Wachtveitland and A. Heckel, Org. Lett., 2011, 13, 1450 CrossRef; (b) C. Menge and A. Heckel, Org. Lett., 2011, 13, 4620 CrossRef CAS.
- A. Momotake, N. Lindegger, E. Niggli, R. J. Barsotti and G. C. R. Ellis-Davies, Nat. Methods, 2006, 3, 35 CrossRef CAS.
- H. Lusic, R. Uprety and A. Deiters, Org. Lett., 2010, 12, 916 CrossRef CAS.
- A. Rodrigues-Correia, P. Seyfried and A. Heckel, in Current Protocols in Nucleic Acid Chemistry, ed. M. Egli, P. Herdewijn, A. Matusda and Y. S. Sanghvi, John Wiley & Sons, Inc., 2014, unit 6.11.1 Search PubMed.
- U. Endesfelder, S. Malkusch, F. Fricke and M. Heilemann, Histochem. Cell Biol., 2014, 141, 629 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization of the new compounds, one- and two-photon illumination procedures, widefield and super-resolution microscopy details. See DOI: 10.1039/c5cc05700k |
| This journal is © The Royal Society of Chemistry 2015 |