Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Jadomycins, put a bigger ring in it: isolation of seven- to ten-membered ring analogues†
Camilo F.
Martinez-Farina
a and
David L.
Jakeman
*b
aDepartment of Chemistry, Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada
bCollege of Pharmacy, Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada. E-mail: david.jakeman@dal.ca
First published on 12th August 2015
Abstract
We report the production, isolation, and characterization of jadomycins with seven-, nine-, and ten-membered E-rings, all of which are unique natural product ring scaffolds. This significantly expands the scope of a non-enzymatic biosynthetic step in jadomycin biosynthesis in order to produce novel natural products.
Natural products have traditionally been obtained from a variety of sources, including plants, fungi, and bacteria.1,2 Bacteria have long been prolific sources of novel natural products, with the Actinomycete genus Streptomyces being responsible for approximately 32% of all bioactive metabolites.3,4 The discovery of novel natural products is important both medically, through the discovery of new clinically active metabolites, and chemically, through the discovery of new chemical scaffolds. This is the case with Streptomyces venezuelae ISP5230, which has been shown to produce baduredin,5 chloramphenicol6 and the jadomycins,7,8 a family of secondary metabolites. From the initial discovery of jadomycin A8 and the glycosylated jadomycin B,9 over twenty-five jadomycins have been isolated.10,11 This has been accomplished through the exploitation of a unique non-enzymatic step in the type II polyketide synthase (PKS) biosynthetic pathway (Scheme 1),12–14 leading to the expansion of the jadomycin library through the incorporation of differing amino acids10 and subsequent chemical derivatization of chemoselective handles.15,16
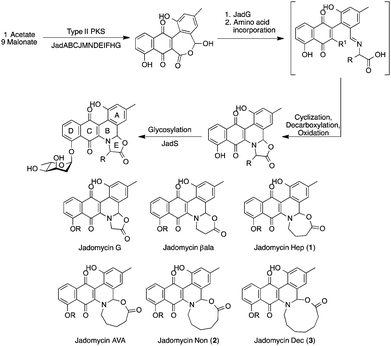 | ||
| Scheme 1 Jadomycin biosynthetic pathway where R is an amino acid side chain and R1 is a proton13 or a carboxylate group14 and structures of ring-expanded variants described herein (R is L-digitoxose). | ||
A novel eight-membered ring-containing jadomycin, was recently observed preferentially over the predicted five-membered oxazolone ring species.15 In order to investigate this further, growths using 4-aminobutanoic acid, 6-aminohexanoic acid, 7-aminoheptanoic acid, and 8-aminooctanoic acid were conducted. These, along with glycine,10 β-alanine,10 and 5-aminopentanoic acid,15 would complete a series of increasing, non-substituted jadomycin E-rings from the five-membered oxazolone ring to the eleven-membered ring jadomycin and would be novel natural product scaffolds.
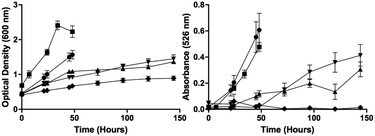
All amino acids investigated allowed for the growth of S. venezuelae ISP5230 VS1099, as determined by OD600 (Fig. 1). However, not all appeared to produce jadomycins, as determined by Abs526 values (Fig. 1). At 48 hours, a typical jadomycin production period, it appeared that production favoured the smaller ring size, with cultures containing 4-aminobutanoic acid having the highest Abs526 values and those with 8-aminooctanoic acid having the lowest Abs526 values. Due to their low Abs526 readings, productions using 6-aminohexanoic acid, 7-aminoheptanoic acid, and 8-aminooctanoic acid were grown for 150 h. At this time-point, only cultures containing 8-aminooctanoic acid failed to produce any appreciable absorbance, suggesting an inability to produce the corresponding jadomycin.
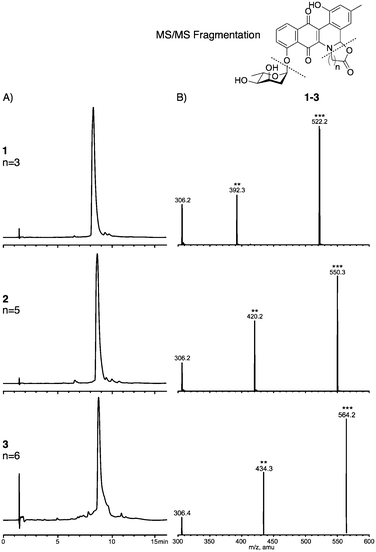
Following an initial phenyl column all jadomycin analogues were present by HPLC and LC-MS/MS, with the exception of jadomycin 8-aminooctanoic acid. A series of chromatographic steps afforded the final natural products (Table 1 and Fig. 2); jadomycin Hep (1, 21 mg L−1), jadomycin Non (2, 10 mg L−1), and jadomycin Dec (3, 3 mg L−1). The amounts isolated and Abs526 values correlate, and are similar to those of previously isolated jadomycins.14,15 The seven-membered ring jadomycin is formed more readily than the eight- or nine-membered ring systems, and the ten-membered ring-containing jadomycin was isolated in the lowest quantity. All jadomycins were isolated as diastereomeric mixtures, arising from the configuration of H3a. Previously, other jadomycins have shown interconversion between the diastereomers, and for this reason, separation of the diastereomers was not attempted.17
| Product | Mj![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mna Mna |
Yield (mg L−1) | HRMS | Calculated mass |
|---|---|---|---|---|
| a Major (Mj) and minor (Mn) H3a diastereomeric ratios were determined by 1H-NMR spectroscopy. b HRMS values obtained and calculated are [M + Na]+. c J. Am. Chem. Soc. 2015, 137, 3271–3275. d Diastereomeric ratio was hard to determine due to signal overlap in the 1H-NMR spectrum. e HRMS values obtained and calculated are [M + H]+. | ||||
| 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
21 | 544.1601 | 544.1578b |
| Jad AVAc | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
10 | 558.1753 | 558.1735b |
| 2 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
10 | 572.1887 | 572.1891b |
| 3 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58d 58d |
3 | 564.2228 | 564.2228e |
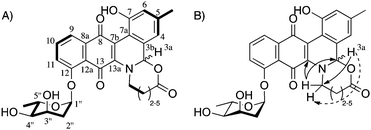
NMR spectroscopy was used to fully characterize the three jadomycin analogues 1–3. 1H–1H COSY NMR established the connectivity of the typical jadomycin spin systems; the A ring, D ring, and sugar ring (Fig. 3A). 1H–13C HMBC NMR established the remaining typical jadomycin connectivity by correlating H1′′ to C12, H9 to C8, H11 to C13, and H3a to C13a. These correlations are similar to those of recently isolated jadomycins.15,18 COSY NMR was also used to identify the presence of the linear amino acids within the jadomycin scaffold (Fig. 3A). HMBC NMR and ROESY NMR were also used to confirm the incorporation of the linear amino acids by correlating H3a to the carbon closest to the nitrogen in the E-ring and H3a to the protons closest to the nitrogen in the E-ring (Fig. 3B). The 1H-NMR chemical shifts of the seven-membered ring of 1 were also similar to those of synthetic 3-tosyl[1,3]oxazepan-7-one,19 providing further evidence to confirm the proposed chemical structure. Collectively, the spectroscopic data demonstrate the isolation of jadomycin analogues containing seven-, nine-, and ten-membered E-rings.
While characterizing the isolated jadomycin analogues, it was noted that the H3a chemical shift in CD3OD changed from the five- (5.18 ppm),9 six- (5.53 ppm, CDCl3),9 seven- (5.64 ppm), eight- (5.64 ppm),14 and nine-membered (5.62 ppm) ring analogues to 4.72 ppm of the ten-membered ring analogue (Fig. 4). This chemical shift is in contrast to previously isolated jadomycins that all have H3a chemical shifts at approximately 5.6 ppm.9,14,15 In addition, the H1 (the protons adjacent to the E-ring carbonyl group) chemical shift changed from the seven- (2.29 ppm), eight- (2.19 ppm),14 and nine-membered (2.24 ppm) ring analogues to 3.44 ppm in the ten-membered ring analogue. Structural changes were also observed through NOESY/ROESY NMR spectroscopy, where irradiation of H3a showed correlations to protons within the eight-, nine-, and ten-membered rings, other than that of the protons adjacent to the nitrogen (Fig. 4), providing evidence of flexibility in the larger ring structures. These correlations were not observed in the ROESY NMR spectrum of the smaller seven-membered ring analogue (Fig. 4). The combination of chemical shift changes and NOE differences provide a rationale for the 30 nm change in the λmax observed between jadomycin Hep (1, 524 nm), jadomycin AVA (522 nm), and jadomycin Non (2, 522 nm) as compared to jadomycin Dec (3, 551 nm). This is consistent with the change in colour that was observed for the ten-membered jadomycin analogue.
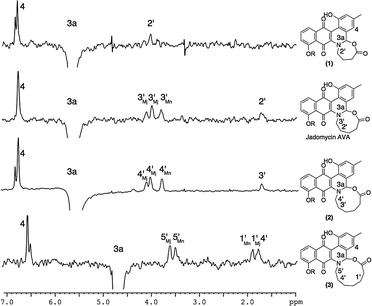 | ||
| Fig. 4 Overlaid ROESY spectra of compounds 1–3 and NOESY spectrum of jadomycin AVA showing the irradiation of H3a and subsequent correlations observed. | ||
The newly isolated jadomycin analogues 1–3, together with jadomycin AVA, enables the effect of increasing the size and lipophilicity of the jadomycin E-ring on the biological activity. These data are being collected in collaboration with the National Cancer Institute (USA) and will be reported in due course.
The production and isolation of compounds 1–3 are the first examples of jadomycin analogues incorporating seven-, nine-, and ten-membered ring structures. These compounds demonstrate new opportunities for non-enzymatic incorporation with linear amino acids of differing chain lengths. Whilst the process of amino acid incorporation is non-enzymatic, the fact that incorporation of 8-aminooctanoic acid was unsuccessful in producing the jadomycin of interest suggests that there are additional factors that determine the scope of reactivity. Investigations into the production of the eleven-membered ring analogue through the use of modified production conditions are being explored, and, if successful, will be reported in due course.
We acknowledge that this research was funded in part by NSERC, CIHR, and NSHRF. We would like to thank Dr Nadine Merkley and Ian Burton at the NRC-IMB for their NMR support and Xiao Feng for acquisition of HRMS data.
Notes and references
- A. L. Harvey, R. Edrada-Ebel and R. J. Quinn, Nat. Rev. Drug Discovery, 2015, 15, 111 CrossRef PubMed.
- P. J. Rutledge and G. L. Challis, Nat. Rev. Microbiol., 2015, 13, 509 CrossRef CAS PubMed.
- J. Berdy, J. Antibiot., 2005, 58, 1 CrossRef CAS PubMed.
- K. Scherlach and C. Hertweck, Org. Biomol. Chem., 2009, 7, 1753 CAS.
- J. D. Sidda, L. Song, V. Poon, M. Al-Bassam, O. Lazos, M. J. Buttner, G. L. Challis and C. Corre, Chem. Sci., 2014, 5, 86 RSC.
- J. He, N. Magarvey, M. Piraee and L. C. Vining, Microbiology, 2001, 147, 2817 CAS.
- D. L. Jakeman, C. L. Graham, W. Young and L. C. Vining, J. Ind. Microbiol. Biotechnol., 2006, 33, 767 CrossRef CAS PubMed.
- S. W. Ayer, A. G. McInnes, P. Thibault, L. Wang, J. L. Doull, T. Parnell and L. C. Vining, Tetrahedron Lett., 1991, 32, 6301 CrossRef CAS.
- J. L. Doull, S. W. Ayer, A. K. Singh and P. Thibault, J. Antibiot., 1993, 46, 869 CrossRef CAS.
- C. N. Borissow, C. L. Graham, R. T. Syvitski, T. R. Reid, J. Blay and D. L. Jakeman, ChemBioChem, 2007, 8, 1198 CrossRef CAS PubMed.
- D. L. Jakeman, C. N. Borissow, T. R. Reid, C. L. Graham, S. C. Timmons and R. T. Syvitski, Chem. Commun., 2006, 3738 RSC.
- U. Rix, J. Zheng, L. L. Remsing Rix, L. Greenwell, K. Yang and J. Rohr, J. Am. Chem. Soc., 2004, 126, 4496 CrossRef CAS PubMed.
- M. Shan, E. U. Sharif and G. A. O'Doherty, Angew. Chem., Int. Ed., 2010, 49, 9492 CrossRef CAS PubMed.
- M. K. Kharel and J. Rohr, Curr. Opin. Chem. Biol., 2012, 16, 150 CrossRef CAS PubMed.
- A. W. Robertson, C. Martinez-Farina, D. A. Smithen, H. Yin, S. Monro, A. Thompson, S. A. Mcfarland, R. T. Syvitski and D. L. Jakeman, J. Am. Chem. Soc., 2015, 137, 3271 CrossRef CAS PubMed.
- S. N. Dupuis, A. W. Robertson, T. Veinot, S. M. A. Monro, S. E. Douglas, R. T. Syvitski, K. B. Goralski, S. A. McFarland and D. L. Jakeman, Chem. Sci., 2012, 3, 1640 RSC.
- R. T. Syvitski, C. N. Borissow, C. L. Graham and D. L. Jakeman, Org. Lett., 2006, 8, 697 CrossRef CAS PubMed.
- C. F. Martinez-Farina, A. W. Robertson, H. Yin, S. M. A. Monro, S. A. McFarland, R. T. Syvitski and D. L. Jakeman, J. Nat. Prod., 2015, 78, 1208 CrossRef CAS PubMed.
- M. Chang, S. Wang and C. Pai, Tetrahedron Lett., 2006, 47, 6389 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, supporting figures, NMR spectra, and characterization data are described. See DOI: 10.1039/c5cc05571g |
| This journal is © The Royal Society of Chemistry 2015 |