 Open Access Article
Open Access ArticleDendritic nanofibers of gold formed by the electron transfer at the interface between water and a highly hydrophobic ionic liquid†
Naoya
Nishi
*,
Tatsuya
Kakinami
and
Tetsuo
Sakka
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto 615-8510, Japan. E-mail: nishi.naoya.7e@kyoto-u.ac.jp
First published on 20th July 2015
Abstract
Gold nanofibers have been found to be formed via a heterogeneous electron-transfer reaction at the ionic liquid|water interface. The tips of the nanofibers show a dendritic structure and the dendrites are bundled to nanofibers except around the tips. The roles of the ionic liquid for the dendritic nanofiber formation have been discussed.
Gold nanostructures have specific optical, electrical, and catalytic properties different from those in the bulk and have been extensively studied for their application in catalysts, sensors, and bioimaging.1,2 The formation of gold nanostructures has been effectively performed by the reduction of Au ions such as AuCl4− by a reducing agent. For the reaction media of the reduction, oil(O)–water(W) two-phase systems have been utilized since more than a century3 and such studies have boomed after the development of the “Brust–Schiffrin” method for the formation of Au nanoparticles.4 Among such two-phase systems for the Au nanostructure formation, the reaction can be spatially restricted at the O|W interface, e.g., by using a metal ion soluble only in one phase and a reducing agent soluble only in the other phase.5 For the Au deposition at the O|W interface, we may choose a combination of the metal ion and the reducing agent that leads to either of the spontaneous reaction or the reaction driven by the externally-controlled phase-boundary potential across the interface. The latter has been adopted to control and analyze the kinetics of reduction at the O|W interface.6–9 The most often studied Au nanostructures formed at the O|W interface are nanoparticles, but more complicated Au nanostructures have also been found to be formed at the O|W interface. Rao et al. prepared 2-D fractal and dendritic nanostructures formed by Au “nanocauliflowers” at the toluene|W interface in the presence of tetraoctylammonium bromide (TOAB) or cetyltrimethylammonium bromide (CTAB),10 which is one of the results of their extensive studies for the preparation of metal nanostructures using the oil–water two-phase system.11 Soejima et al. utilized linear aggregates of tetrabutylammonium Au(OH)4− ion pairs formed at the chloroform|W interface for the photoreductive formation of holey Au nanowires.12 In the present study, we will introduce another nanostructure of dendritic nanofibers of gold, which is formed by the electron transfer at another kind of liquid–liquid interface where we used a highly hydrophobic ionic liquid (IL) instead of molecular organic solvents (oil).
ILs are liquid salts that are entirely composed of ions and are promising materials for replacing conventional organic solvents in many scientific fields.13–15 When ILs are composed of hydrophobic cations and anions the ILs are immiscible with W16 and furthermore when the IL-ions are highly hydrophobic the IL|W interface is electrochemically polarizable.17,18 The development of highly hydrophobic ILs has enabled us to perform electrochemical measurements at the IL|W interface,19,20 using the same methodology as that at the O|W interface.21–23 For example, the transfer of ions17,18,24–30 and the facilitated transfer of metal ions in W by a ligand in the IL31–34 across the IL|W interface have been studied. The electron transfer across the IL|W interface has also been studied between redox species in IL and those in W,35–39 which prompted us to measure electron-transfer current due to the electrodeposition of metal ions at the IL|W interface in the present study.
Aside from the viewpoint of electrochemistry at the liquid–liquid interface, ILs have some characteristics that provide benefits for the formation of unique metal nanostructures. First, ILs have high viscosity, typically hundreds of times higher than W, which leads to slow mass transfer of the reactants in ILs for the metal deposition. This will induce a significant imbalance of the mass transfer rates from the W side and the IL side of the IL|W interface where the metal ion and the reducing agent react and such mass transfer imbalance has been discussed as a key factor to prepare the 1-D nanostructure of the metal at the O|W interface.40–42 Second, ILs form a specific structure, ionic multilayers at interfaces.43,44 By virtue of this property researchers have successfully prepared well-dispersed metal nanoparticles in ILs without any additives to prevent aggregation.45 This remarkable stabilization of metal nanoparticles in ILs has been discussed in the viewpoint of the ability of IL-ions acting as “capping reagents”.45 Regarding the role of IL-ions as the capping reagent, we may also anticipate the influence of IL ions on the final metal nanostructures because some IL-ions are surface-active but others are not. In fact, Yao et al. recently prepared Ag films by reducing Ag+ in W with a reducing agent in the IL at the IL|W interface, whose morphologies depend on the used IL-ions.46 Finally, ILs are regarded as dense electrolyte solutions with an ionic strength of several mol dm−3 and thus possess the ability to screen the electric field (i.e. thin electrical double layer). This screening ability is expected to make metal nanostructures in ILs assemble easily, without aggregation because of the capping ability described above. In the present study, we will show that the formation of Au nanofibers becomes possible because of the above-mentioned characteristics of ILs.
The highly hydrophobic IL used in the present study, trioctylmethylammonium bis(nonafluorobutanesulfonyl)amide ([TOMA+][C4C4N−]), was prepared47 and purified48,49 in the same way as in the literature. In [TOMA+][C4C4N−] tri-p-tolylamine (TPTA, TCI) was dissolved as a reducing agent. AuCl3 (Shimadzu) was dissolved in an aqueous solution of HCl to form AuCl4− in W as a source of metal (precursor). The details of the electrochemical measurements for the ion and electron transfer at the IL|W interface and scanning electron microscopy (SEM) analysis of the Au deposits are given in the ESI.†
Fig. 1a shows cyclic voltammograms at the IL|W interface using Cell I (see ESI†) in the absence of TPTA in the IL (x = 0, where x denotes the molality of TPTA in the IL in the unit of mmol kg−1). Without TPTA, the reducing agent in the present study, no electron transfer occurs at the IL|W interface and the ion transfer across the IL|W interface is detected as current. For the case with (x, y) = (0, 0) (red curve in Fig. 1a), also in the absence of AuCl4−, one can see potential windows with a width of 500 mV47 (where y denotes the molarity of AuCl4− in W in the unit of mmol dm−3). In the presence of AuCl4− in W, cyclic voltammograms with (x, y) = (0, 1) (blue) and (0, 10) (green) show negative current at the forward scan and positive current at the backward scan. The negative and positive currents reflect the transfer of AuCl4− from W to IL and from IL to W, respectively, as was previously observed at the O|W interface.5,50–53
| AuCl4−(W) ⇌ AuCl4−(IL) | (1) |
The shape of voltammograms is not as simple as those limited by linear or radial mass transfer, because of the truncated conical geometry of the micropipette tip and also the small diffusion coefficient of the ion outside the micropipette caused by the high viscosity of the IL (2000 mPa s at 25.0 °C).47,54 With a slower scan (e.g. 0.1 mV s−1) and a smaller micropipette tip (2 μm) we would record sigmoidal voltammograms, judging from previous experimental24 and simulation54 results. In such a complicated case we cannot precisely determine the mid-point potential (or half-wave potential) for the transfer of AuCl4−; however, we roughly estimate it to be −250 mV.
In order to focus on the electron transfer at the IL|W interface, we used the ECSOW system where the liquid–liquid interface is separated by an electron conductor to prevent the ion transfer.53,55 In our case, the Au wire and the Pt microelectrode, which were electrically connected, were immersed into W and IL, respectively (see the ESI† for details). Fig. 1b shows the electron-transfer cyclic voltammogram, where the current is limited by the mass transfer and redox of TPTA+/TPTA. One can see the semi-infinite linear diffusion limited voltammogram having the positive and negative current peaks, indicating the simple redox of TPTA.52,56
| TPTA(IL) ⇌ TPTA+(IL) + e− | (2) |
| AuCl4−(W) + 3TPTA(IL) ⇌ Au(σ) + 4Cl−(W) + 3TPTA+(IL) | (3) |
| AuCl4−(W) + 3TPTA(IL) + 3AuCl4−(W) ⇌ Au(σ) + 4Cl−(W) + 3TPTA+(IL) + 3AuCl4−(IL) | (4) |
To obtain an analyzable amount of the deposit for the morphology analysis, the deposit was prepared at the macro IL|W interface with an area of 1.1 cm2. After the contact of the W phase with the IL phase, the formation of gold-coloured deposits at the IL|W interface was observed by the naked eye. The SEM images for the deposit are shown in Fig. 2a and b. The deposits are highly anisotropic 1-D nanostructures whose diameter is 600 nm on an average and whose length is from 10 to 30 μm (Fig. 2a). The tips of the nanostructures have a feather-like dendritic structure whose trunks and branches are 50–100 nm in size (Fig. 2b). The dendritic structures are bundled to nanofibers except around the tips (Fig. 2a and b), keeping small gaps between trunks and branches in the bundles. It should be noted here that these structures are observed using ex situ SEM and may be somewhat different from those present at the IL|W interface. The EDX analysis performed simultaneously with SEM measurements only detected X-ray fluorescence of Au without signals from other elements, illustrating that the nanofibers are made of the Au metal (see the ESI† for the EDX images). To check the existence of any peculiar roles of the IL for the Au nanofiber formation, we performed similar experiments using dichloromethane (DCM), an organic solvent, instead of the IL. The nanostructure obtained at the DCM|W interface is shown in Fig. 2c. We obtained burr-like structures in the case of DCM. The results indicate that gold nanofibers are unique structures that are grown peculiarly at the IL|W interface.
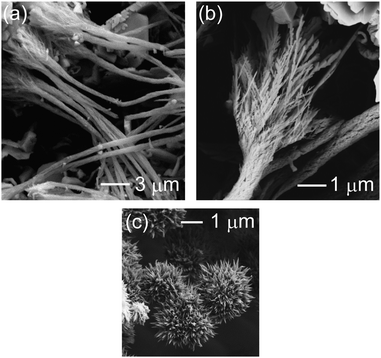 | ||
| Fig. 2 SEM images (a and b) for the Au nanofibers formed at the IL|W interface and (c) for the Au nanostructures formed at the DCM|W interface. | ||
One of the questions is why such dendritic structures are formed around the tips of the Au nanofibers. To form a dendritic nanostructure of Au, previous studies designed chemical species that play a critical role in the reaction. In the case of homogeneous Au reduction in aqueous solutions by a reducing agent,59–61 customized cationic surfactants, tetraalkylammonium bromides, were added in the solutions to prepare Au dendrites, inspired by the famous capping reagent CTAB used in Au nanorod synthesis.1 Electrodeposition at the glassy carbon electrode also leads to Au dendrites in the presence of cysteine.62 Qin et al. successfully prepared dendritic Au at the interface of Zn and 1-butyl-3-methylimidazolium hexafluorophosphate, an IL, where Zn metal acts as a reducing agent.63 All the above reports used their special chemical species to fulfil the Au dendrite formation. Therefore, either or both the presence of TOMA+ (a tetraalkylammonium cation) and C4C4N−, the IL ions used in the present study, at the Au surface probably play a crucial role in the dendrite formation in the present study, although the reduction site at the Au surface is likely to be at the W side of the IL|W interface. TOMA+ and C4C4N− are both surface-active and are expected to be adsorbed at the surface of Au nanostructures, which is presumably preferential adsorption at the particular crystal faces, as was proposed for CTAB adsorption at the surface of Au nanorods.1
Another question is why we obtained highly anisotropic 1-D nanostructures at the IL|W interface. The reason can be discussed taking into account the results in previous papers that created Ag nanowires at the O|W interface.40–42 Scholz and Hasse suggested that an important factor for the 1-D structure formation is the ratio of mass transfer of Ag+ ions in W, fW, to that of reducing agents in O, fO, toward the Ag surface at the O|W interface.40 The reduction of Ag+ ions and the oxidation of the reducing agent occur simultaneously but not necessarily at the same place of the Ag surface because Ag nanowires having electron conductivity can deliver electrons from one surface site to another. If fO ≪ fW, which is achieved by adjusting the ratio of the initial concentrations of both species, the Ag metal at the O|W interface “grows” to the O phase to increase the area of the Ag surface and the volume of diffusion zones on the O side, consequently balancing the two mass transfer rates. They actually confirmed that the Ag nanowires were formed when the concentration of Ag+ ion in W is significantly higher than that of the reducing agent in O.40 This is likely to be the case with the formation of the Au nanofibers. In the present study, the concentration of TPTA in the IL, 40 mmol kg−1, is comparable to (even higher than) the concentration of AuCl4− in W, 10 mM. Nevertheless, the high viscosity of the IL decelerates the mass transfer on the IL side by the factor of 2200 (ηIL/ηW where η is the viscosity). This imbalance of the mass transfer is reduced by the growth of Au nanofibers into the IL, enlarging the surface area for the oxidation of TPTA.
We also observed Au nanofibers using in situ optical microscopy (see the ESI† for the images). The images show μm-scale bright spots that seems to be mesoscale assembly of Au nanofibers. Such mesoscale assembly was also observed for Au nanoparticles formed at the oil–water interface in previous optical microscopy studies.64,65 It seems that Au nanofibers remain around the interface in the IL and form the mesoscale assembly, providing the large reaction surface for the TPTA oxidation.
Finally, the bundle of the Au nanofibers (Fig. 2a and b) except around the tips seems to be due to the greater ability of ILs to screen electrostatic repulsion than W. ILs are considered as dense “ionic solutions”, having a short Debye length for an electrical double layer on the order of 0.1 nm (estimated in the framework of mean-field theory). We speculate that the Au dendrites formed on the W side of the IL|W interface move across the interface to the IL side due to the imbalance of the mass transfer (see the above discussion), where the electrostatic repulsion between the Au surfaces lessens and the dendrites are bundled to the Au nanofibers. The nanofibers are bundled but not aggregated possibly because IL-ions are forming a rigid structure at the surface of the nanofibers, preventing the contact of two surfaces.45
In conclusion, we prepared dendritic nanofibers of gold by the reduction of Au ions at the IL|W interface. The formation of this novel nanostructure became possible because of several characteristics of ILs and IL-ions: high viscosity, specific surface structure, and high ionic strength. The dendritic nanofibers of gold possess numerous structural defects, a high surface area and narrow gaps inside the nanofibers, appealing to the applications to catalysts and SERS-based sensors. Future studies include the formation of a similar nanostructure of other metals such as Ag, Pt, and Pd, and the fine control of the nanofiber structures by changing IL-ions and reducing agents as well as the applications to catalysts and SERS materials, and such studies are in progress in our laboratory.
This work was partly supported by a Grant-in-Aid for Scientific Research (No. 26410149) and by the Hattori-Hokokai Foundation.
Notes and references
- S. E. Lohse and C. J. Murphy, Chem. Mater., 2013, 25, 1250 CrossRef CAS
.
- N. Li, P. Zhao and D. Astruc, Angew. Chem., Int. Ed., 2014, 53, 1756 CrossRef CAS PubMed
.
- M. Faraday, Philos. Trans. R. Soc. London, 1857, 147, 145 CrossRef
.
- M. Brust, M. Walker, D. Bethell, D. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801 RSC
.
- Y. Cheng and D. Schiffrin, J. Chem. Soc., Faraday Trans., 1996, 92, 3865 RSC
.
- C. Johans, R. Lahtinen, K. Kontturi and D. Schiffrin, J. Electroanal. Chem., 2000, 488, 99 CrossRef CAS
.
- R. A. W. Dryfe, Phys. Chem. Chem. Phys., 2006, 8, 1869 RSC
.
- A. Trojánek, J. Langmaier and Z. Samec, J. Electroanal. Chem., 2007, 599, 160 CrossRef PubMed
.
- R. A. W. Dryfe, A. Uehara and S. G. Booth, Chem. Rec., 2014, 14, 1013 CrossRef CAS PubMed
.
- V. V. Agrawal, G. U. Kulkarni and C. N. R. Rao, J. Colloid Interface Sci., 2008, 318, 501 CrossRef CAS PubMed
.
- C. N. R. Rao and K. P. Kalyanikutty, Acc. Chem. Res., 2008, 41, 489 CrossRef CAS PubMed
.
- T. Soejima, M. Morikawa and N. Kimizuka, Small, 2009, 5, 2043 CrossRef CAS PubMed
.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC
.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef CAS PubMed
.
- M. V. Fedorov and A. A. Kornyshev, Chem. Rev., 2014, 114, 2978 CrossRef CAS PubMed
.
- J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 1765 RSC
.
- T. Kakiuchi and N. Tsujioka, Electrochem. Commun., 2003, 5, 253 CrossRef CAS
.
- H. Katano and H. Tatsumi, Anal. Sci., 2003, 19, 651 CrossRef CAS
.
- T. Kakiuchi, N. Tsujioka, K. Sueishi, N. Nishi and M. Yamamoto, Electrochemistry, 2004, 72, 833 CAS
.
- Z. Samec, J. Langmaier and T. Kakiuchi, Pure Appl. Chem., 2009, 81, 1473 CrossRef CAS
.
-
R. A. W. Dryfe, in Advances in Chemical Physics, ed. S. A. Rice, Wiley, New York, 2009, vol. 141, p. 153 Search PubMed
.
-
H. H. Girault, in Electroanalytical Chemistry, ed. A. J. Bard and C. G. Zoski, CRC Press, Boca Raton, 2010, vol. 23, p. 1 Search PubMed
.
- Z. Samec, Electrochim. Acta, 2012, 84, 21 CrossRef CAS PubMed
.
- N. Nishi, S. Imakura and T. Kakiuchi, Anal. Chem., 2006, 78, 2726 CrossRef CAS PubMed
.
- V. A. Hernández and F. Scholz, Electrochem. Commun., 2006, 8, 967 CrossRef PubMed
.
- J. Langmaier and Z. Samec, Electrochem. Commun., 2007, 9, 2633 CrossRef CAS PubMed
.
- Y. Wang, T. Kakiuchi, Y. Yasui and M. V. Mirkin, J. Am. Chem. Soc., 2010, 132, 16945 CrossRef CAS PubMed
.
- D. S. Silvester and D. W. M. Arrigan, Electrochem. Commun., 2011, 13, 477 CrossRef CAS PubMed
.
- J. Langmaier, Z. Samec, E. Samcová and P. Tůma, Electrochem. Commun., 2012, 24, 25 CrossRef CAS PubMed
.
- T. J. Stockmann, A.-M. Montgomery and Z. Ding, J. Electroanal. Chem., 2012, 684, 6 CrossRef CAS PubMed
.
- N. Nishi, H. Murakami, S. Imakura and T. Kakiuchi, Anal. Chem., 2006, 78, 5805 CrossRef CAS PubMed
.
- J. Langmaier and Z. Samec, Anal. Chem., 2009, 81, 6382 CrossRef CAS PubMed
.
- T. J. Stockmann, Y. Lu, J. Zhang, H. H. Girault and Z. Ding, Chem. – Eur. J., 2011, 17, 13206 CrossRef CAS PubMed
.
- T. J. Stockmann, J. Zhang, A.-M. Montgomery and Z. Ding, Anal. Chim. Acta, 2014, 821, 41 CrossRef CAS PubMed
.
- F. O. Laforge, T. Kakiuchi, F. Shigematsu and M. V. Mirkin, J. Am. Chem. Soc., 2004, 126, 15380 CrossRef CAS PubMed
.
- F. O. Laforge, T. Kakiuchi, F. Shigematsu and M. V. Mirkin, Langmuir, 2006, 22, 10705 CrossRef CAS PubMed
.
- J. Langmaier, A. Trojánek and Z. Samec, Electrochem. Commun., 2010, 12, 1333 CrossRef CAS PubMed
.
- J. Langmaier and Z. Samec, Electrochim. Acta, 2011, 58, 606 CrossRef CAS PubMed
.
- J. Jedraszko, W. Nogala, W. Adamiak, S. Dongmo, G. Wittstock, H. H. Girault and M. Opallo, Chem. Commun., 2015, 51, 6851 RSC
.
- F. Scholz and U. Hasse, Electrochem. Commun., 2005, 7, 541 CrossRef CAS PubMed
.
- K. Luo and R. A. W. Dryfe, New J. Chem., 2009, 33, 157 RSC
.
- U. Hasse, G. J. Palm, W. Hinrichs, J. Schäfer and F. Scholz, Phys. Chem. Chem. Phys., 2011, 13, 12254 RSC
.
- M. Mezger, H. Schröder, H. Reichert, S. Schramm, J. S. Okasinski, S. Schöder, V. Honkimäki, M. Deutsch, B. M. Ocko, J. Ralston, M. Rohwerder, M. Stratmann and H. Dosch, Science, 2008, 322, 424 CrossRef CAS PubMed
.
- N. Nishi, Y. Yasui, T. Uruga, H. Tanida, T. Yamada, S. Nakayama, H. Matsuoka and T. Kakiuchi, J. Chem. Phys., 2010, 132, 164705 CrossRef PubMed
.
- T. Torimoto, T. Tsuda, K.-i. Okazaki and S. Kuwabata, Adv. Mater., 2010, 22, 1196 CrossRef CAS PubMed
.
- K. Yao, W. Lu, X. Li, J. Wang and J. Yuan, Chem. Commun., 2013, 49, 1398 RSC
.
- N. Nishi, H. Murakami, Y. Yasui and T. Kakiuchi, Anal. Sci., 2008, 24, 1315 CrossRef CAS
.
- M. J. Earle, C. M. Gordon, N. V. Plechkova, K. R. Seddon and T. Welton, Anal. Chem., 2007, 79, 758 CrossRef CAS PubMed
.
- Y. Yasui, Y. Kitazumi, R. Ishimatsu, N. Nishi and T. Kakiuchi, J. Phys. Chem. B, 2009, 113, 3273 CrossRef CAS PubMed
.
- R. Knake, A. Fahmi, S. A. M. Tofail, J. Clohessy, M. Mihov and V. J. Cunnane, Langmuir, 2005, 21, 1001 CrossRef CAS PubMed
.
- Y. Gründer, H. L. T. Ho, J. F. W. Mosselmans, S. L. M. Schroeder and R. A. W. Dryfe, Phys. Chem. Chem. Phys., 2011, 13, 15681 RSC
.
- Y. Gründer, J. F. W. Mosselmans, S. L. M. Schroeder and R. A. W. Dryfe, J. Phys. Chem. C, 2013, 117, 5765 Search PubMed
.
- A. Uehara, T. Hashimoto and R. A. W. Dryfe, Electrochim. Acta, 2014, 118, 26 CrossRef CAS PubMed
.
- N. Nishi, S. Imakura and T. Kakiuchi, J. Electroanal. Chem., 2008, 621, 297 CrossRef CAS PubMed
.
- H. Hotta, N. Akagi, T. Sugihara, S. Ichikawa and T. Osakai, Electrochem. Commun., 2002, 4, 472 CrossRef CAS
.
- M. Planells, L. X. Reynolds, U. Bansode, S. Chhatre, S. Ogale, N. Robertson and S. A. Haque, Phys. Chem. Chem. Phys., 2013, 15, 7679 RSC
.
- J. Guo, T. Tokimoto, R. Othman and P. R. Unwin, Electrochem. Commun., 2003, 5, 1005 CrossRef CAS PubMed
.
- X. Zhu, Y. Qiao, X. Zhang, S. Zhang, X. Yin, J. Gu, Y. Chen, Z. Zhu, M. Li and Y. Shao, Anal. Chem., 2014, 86, 7001 CrossRef CAS PubMed
.
- T. Huang, F. Meng and L. Qi, Langmuir, 2010, 26, 7582 CrossRef CAS PubMed
.
- D. Huang, Y. Qi, X. Bai, L. Shi, H. Jia, D. Zhang and L. Zheng, ACS Appl. Mater. Interfaces, 2012, 4, 4665 CAS
.
- D. Huang, X. Bai and L. Zheng, J. Phys. Chem. C, 2011, 115, 14641 CAS
.
- T.-H. Lin, C.-W. Lin, H.-H. Liu, J.-T. Sheu and W.-H. Hung, Chem. Commun., 2011, 47, 2044 RSC
.
- Y. Qin, Y. Song, N. Sun, N. Zhao, M. Li and L. Qi, Chem. Mater., 2008, 20, 3965 CrossRef CAS
.
- A. I. Campbell, R. A. W. Dryfe and M. D. Haw, Anal. Sci., 2009, 25, 307 CrossRef CAS
.
- S. Tsukahara, R. Kobayashi and T. Fujiwara, Solvent Extr. Res. Dev., Jpn., 2014, 21, 119 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, EDX images, and optical microscopy images. See DOI: 10.1039/c5cc05476a |
| This journal is © The Royal Society of Chemistry 2015 |

