Open Access Article
Open Access ArticleFirst homoleptic MIC and heteroleptic NHC–MIC coordination cages from 1,3,5-triphenylbenzene-bridged tris-MIC and tris-NHC ligands†‡
Carmen
Mejuto
a,
Gregorio
Guisado-Barrios
*a,
Dmitry
Gusev
b and
Eduardo
Peris
*a
aInstituto de Materiales Avanzados (INAM), Universitat Jaume I, Avda. Vicente Sos Baynat, 12071-Castellón, Spain. E-mail: guisado@uji.es; eperis@uji.es
bDepartment of Chemistry and Biochemistry, Wilfrid Laurier University, Waterloo, Ontario N2L 3C5, Canada
First published on 24th July 2015
Abstract
The preparation of a triphenylbenzene-bridged tris-(1,2,3-triazolium) salt allowed us to obtain the first homoleptic tris-MIC cylinder-like cages of Ag and Au. The silver MIC-based cage reacts with the tris-NHC-Ag analogue to form the corresponding heteroleptic NHC–MIC silver cage in an unusual reaction involving the simultaneous exchange of the tris-NHC and tris-MIC ligands.
As defined by Constable in 1994, metallosupramolecular chemistry involves the use of combinations of bridging organic ligands and metal units to synthesize discrete or polymeric assemblies.1 The basis for the construction of metallosupramolecular structures are multitopic ligands with two or more binding domains separated by spacers, which, in combination with suitable metal fragments may form assemblies with various shapes and sizes.2 Most of the known multitopic ligands are based on N or O donor atoms, and some of the most relevant advances in metallosupramolecular chemistry are associated with the names of Fujita,3 Raymond,4 Stang,5 Nitschke,2a,c,6 and Constable,2b,7 among others.8 Extended poly-N-heterocyclic-carbene (NHC) ligands9 have recently been shown to be suitable scaffolds for the preparation of supramolecular assemblies by featuring only metal–carbon bonds. The topological diversity of poly-NHCs has allowed the rapid preparation of a variety of supramolecular assemblies that include molecular squares and triangles,10 cylinder-like structures11 and organometallic polymers.12
Parallel to the rapid growth of NHCs (normally imidazolylidenes), mesoionic carbenes (MICs)13 have recently emerged as a powerful subclass of NHCs due to their ease of accessibility and their arguably stronger electron donor properties compared to the normal NHC-analogues.14 While monometallic complexes bearing mono-MIC ligands are widely used, poly-MIC ligands are not common,15 and this explains why MIC-based supramolecular assemblies are still unknown.
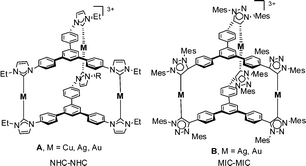
We recently reported the preparation of a C3-symmetric tris-NHC ligand based on a 1,3,5-triphenylbenzene core, which we coordinated to rhodium and iridium.16 The same ligand was used for the preparation of nanometer-sized cylinder-like structures of Cu(I), Ag(I) and Au(I)11g with a hollow central cavity (A, Scheme 1). Based on these findings, we pursued the preparation of the related triphenylbenzene-bridged tris-MIC ligand, to obtain the corresponding molecular cages using a metal-controlled self-assembly methodology. Herein we report an effective preparation of a 1,3,5-triphenylbenzene-bridged tris-azolium salt that was used for the self-assembly formation of the related supramolecular cylinder-like MIC-based compounds of Ag(I) and Au(I) (B, Scheme 1).
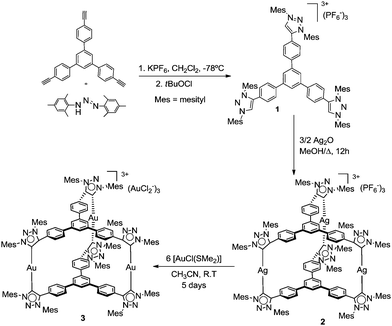
The tris-triazolium salt 1 was obtained in 93% yield, by following the procedure depicted in Scheme 2. The salt was characterized by means of NMR spectroscopy, mass spectrometry and elemental analysis. The 1H and 13C NMR spectra of 1 confirmed the threefold symmetry of the compound. The triazolium C–H resonance appeared at δ 9.08 in the 1H NMR spectrum. The 13C NMR spectrum showed the resonance of the C–H carbon of the triazolium rings at 131.3 ppm.
The reaction of 1 with 1.5 equivalents of Ag2O in MeOH at 60 °C under the exclusion of light, afforded the tris-silver hexa-MIC complex 2 in 42% yield. This compound contains three silver atoms sandwiched between two triphenylbenzene-tris-MIC ligands. The 1H NMR spectrum of 2 indicates that the compound is highly symmetric, as shown by the appearance of four signals due to the protons of the methyls at the mesityl groups (four signals with relative integrals 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36). The 13C NMR spectrum shows the resonance due to the equivalent metallated carbene–carbon atoms at 169.7 ppm, exhibiting the coupling to both silver isotopes (1JC–Ag107 = 165.0 Hz, 1JC–Ag109 = 190.5 Hz). The ESI mass spectrum shows the most intense peak at m/z 919.2, assigned to [M + PF6]2+.
36). The 13C NMR spectrum shows the resonance due to the equivalent metallated carbene–carbon atoms at 169.7 ppm, exhibiting the coupling to both silver isotopes (1JC–Ag107 = 165.0 Hz, 1JC–Ag109 = 190.5 Hz). The ESI mass spectrum shows the most intense peak at m/z 919.2, assigned to [M + PF6]2+.
The reaction of 2 with six equivalents of [AuCl(SMe2)] in acetonitrile at room temperature for 5 days led to the formation of the trinuclear hexa-MIC complex 3 as an air-stable white solid, in 63% yield. The use of excess of Au allows the formation of 3 with three (AuCl2)− counter-anions. For this complex the 1H NMR spectrum also confirms the threefold symmetry of the molecule (four signals are seen for the protons of the methyls at the mesityl groups, with relative intensities 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The 13C NMR spectrum shows the diagnostic signals due to the equivalent carbene carbons at 161.1 ppm. The trimetallic nature of the complex was further confirmed by the ESI mass spectrum, which showed the main peak at m/z 1645.6, attributed to [M + (AuCl2)]2+.
1). The 13C NMR spectrum shows the diagnostic signals due to the equivalent carbene carbons at 161.1 ppm. The trimetallic nature of the complex was further confirmed by the ESI mass spectrum, which showed the main peak at m/z 1645.6, attributed to [M + (AuCl2)]2+.
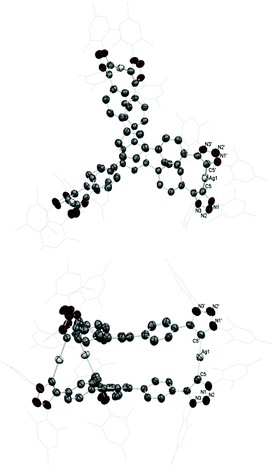
The molecular structure of 2 was confirmed using X-ray diffraction studies (Fig. 1). The molecule consists of two tris-MIC ligands sandwiching the three silver atoms, thus rendering a hexacarbene complex cation, with three PF6− counter-anions. The average distance of the Ag–CMIC bonds is 2.105(7) Å, and the CMIC–Ag–CMIC angle is 177.0°. The distance between the two central benzene rings is 4.318 Å. The three silver atoms form a triangle, with an Ag–Ag separation of about 13.96 Å.
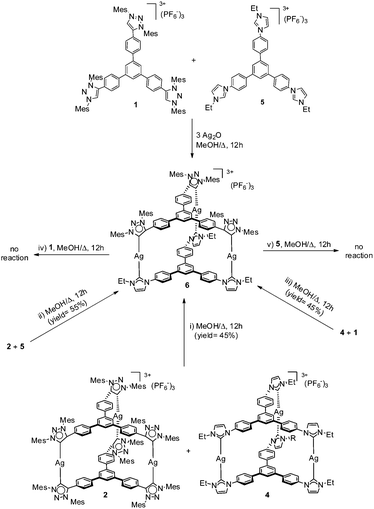
Due to the similarities between the metric parameters of 3, to those shown by the silver complex with the related triphenylbenzene-bridged-tris-NHC ligand (4, Scheme 3),11g we decided to see if we could obtain a cylinder-type coordination complex combining the presence of both tris-NHC and tris-MIC ligands. The preparation of such a type of complex is very interesting because it would allow us to obtain for the very first time a coordination cage with two different types of carbenes. In order to obtain such a compound, we decided to react an equimolar amount of the tris-triazolium salt 1, with the tris-imidazolium salt 5, together with three equivalents of Ag2O in MeOH at 60 °C for 12 hours. In principle, this reaction should allow us to detect the mixed NHC–MIC cage, just by considering a random distribution of the two ligands over the three metal centers. However, our expectations were exceeded, because the mixed NHC–MIC cage was the only metal complex detected in the ESI-mass spectrum of the reaction mixture (a main peak at m/z 1136.8 was observed, assigned to [M + PF6]2+). Moreover, the purification of the resulting metal complex allowed us to obtain the mixed NHC–MIC cage (6, Scheme 3) in 45% yield, thus close to the yield that we should have expected for the formation of this compound, had we assumed a random distribution of the two ligands about the trimetallic structure (50%).
The mixed NHC–MIC silver-based coordination cage 6 was characterized by NMR spectroscopy and mass spectrometry. The 1H NMR spectrum revealed the presence of the two different carbene ligands, as observed by the appearance of the signals due to the ethyl groups and the methyls from the mesityl groups bound to the tris-NHC and tris-MIC ligands, respectively, and the number of signals is consistent with the threefold symmetry of the complex. The 13C NMR spectrum shows the distinct signals due to the presence of the two types of carbene carbons, both displaying the coupling with the two silver isotopes and therefore appearing as four doublets, two centered at 179.5 (1JC–Ag107 = 177.8 Hz, 1JC–Ag109 = 205.5 Hz), and the other two at 169.5 ppm (1JC–Ag-107 = 177.8 Hz, 1JC–Ag-109 = 168.8 Hz), for the NHC and MIC carbene carbons, respectively. These resonances are practically unshifted compared to the resonances of the di-(tris-MIC) and the di-(tris-NHC) silver complexes 2 and 6.11g
Encouraged by this result, we decided to see if we could also obtain compound 6 by other reactions involving the reorganization of the preformed tris-carbene complexes 4 and 2. All the reactions were carried out in MeOH at 60 °C for 12 h, and the combination of reactants were as follows (Scheme 3): (i) equimolar amounts of the two tris-carbene cages, 2 and 4, (ii) equimolar amounts of 2 and the tris-imidazolium salt 5, (iii) equimolar amounts of 4 and the tris-triazolium salt 1, (iv) equimolar amounts of 6 and the tris-triazolium salt 1, and (v) equimolar amounts of 6 and the tris-imidazolium salt 5. The study of the products resulting from each reaction revealed that reactions (i)–(iii) produced the mixed NHC–MIC cage 6 in 40–50% yield, as the only detected (mass spectrometry) and isolable product. The reactions (iv) and (v) did not show any reorganization of the carbene ligands, and complex 6 was quasi-quantitatively recovered from the reaction mixtures.
These results indicate that, regardless of the thermodynamics of the overall reactions, the mixed MIC–NHC complex 6 may be formed and isolated from all possible reactions involving the combination of tris-carbene ligands (or azolium salts) and silver precursors. The reorganization of the ligand is also kinetically favored, as silver carbenes are often used for carbene transfer processes,17 although in our case, we show an unusual example of carbene transfer affording an intermolecular recombination of ligands. This type of ligand redistribution is related to the one recently described by Albrecht and co-workers who found a spontaneous recombination of carbene-ligands in a series of Au-MIC complexes.18
In order to shed some light on these results, and to determine if the formation of the mixed MIC–NHC complex 6 is thermodynamically favorable compared to 2 and 4, we decided to calculate the thermodynamic parameters of reaction (i) shown in Scheme 3 using M06L DFT calculations. Since good-quality computational modelling of the complete structures 2, 4, and 6 is impossible, the calculations were performed on the mononuclear analogues, the optimized geometries of which are shown in Fig. 2. As an illustration of the validity of the computed model, the calculated structure of [Ag(MIC)2]+ is significantly similar to the local coordination geometry around silver in 2. In particular, the calculated Ag–C distance, 2.10 Å, is close to the experimental distances, 2.105–2.114 Å in 2. A peculiar feature of [Ag(MIC)2]+ is the 38.6° phenyl group twist relative to the carbene plane. In the crystal structure of 2 (Fig. 1), the corresponding C6 rings are similarly rotated by 35.2° and 31.0°, despite a more constrained geometry. In methanol, the formation of the mixed carbene complex, [Ag(NHC)2]+ + [Ag(MIC)2]+ → 2 [Ag(NHC)(MIC)]+, is thermo-neutral: ΔH = 0.0 kcal mol−1, whereas in the less polar benzene this reaction is calculated to be slightly favorable: ΔH = −1.6 kcal mol−1. The calculated Ag–C bond enthalpy is stronger for MIC vs. NHC in [Ag(NHC)(MIC)]+: 44.0 vs. 41.8 kcal mol−1 in methanol.
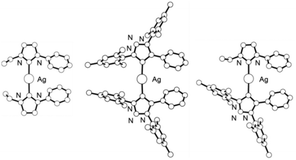 | ||
| Fig. 2 Calculated structures of [Ag(NHC)2]+, [Ag(MIC)2]+, and [Ag(MIC)(NHC)]+ complexes (all hydrogen atoms are omitted for clarity). | ||
Based on the computed energies, the mixed carbene complex is predicted to be the main species under equilibrium conditions, and the reason for being the only isolated complex should be attributed to factors beyond those that we should take into account in DFT calculations (trimetallic nature of the complexes, solubility of the complex, interactions of 2, 4, and 6 with the counter-ions [PF6]−, or explicit interactions with the solvent).
In summary, we have described the first MIC-based molecular cages, and proved that these types of NHC-relatives may be used for the metal-controlled self-assembly of nanometer-sized cylinder-shaped molecules. We were also able to synthesize a unique heteroleptic NHC–MIC cylinder-shaped cage, by the recombination of the ligands in the related MIC- and NHC-based cylinders, and by several other combinations. This reaction involves an unusual rearrangement, which clearly illustrates the lability of the tris-NHC and tris-MIC ligands on silver in solution.
We gratefully acknowledge financial support from MINECO of Spain (CTQ2014-51999-P) and UJI (P11B2014-02). Carmen Mejuto is thankful to the FPI program for a fellowship, and Gregorio Guisado-Barrios thanks the MINECO for a postdoctoral grant (FPDI-2013-16525). The authors are grateful to the Serveis Centrals d'Instrumentació Científica (SCIC) of the Universitat Jaume I for providing with spectroscopic and X-ray facilities.
Notes and references
- E. C. Constable, Chem. Ind., 1994, 56–59 CAS.
- (a) M. M. J. Smulders, I. A. Riddell, C. Browne and J. R. Nitschke, Chem. Soc. Rev., 2013, 42, 1728–1754 RSC; (b) E. C. Constable, Coord. Chem. Rev., 2008, 252, 842–855 CrossRef CAS PubMed; (c) J. R. Nitschke, Acc. Chem. Res., 2007, 40, 103–112 CrossRef CAS PubMed; (d) P. J. Steel, Acc. Chem. Res., 2005, 38, 243–250 CrossRef CAS PubMed; (e) F. Wurthner, C. C. You and C. R. Saha-Moller, Chem. Soc. Rev., 2004, 33, 133–146 RSC.
- (a) M. Fujita, M. Tominaga, A. Hori and B. Therrien, Acc. Chem. Res., 2005, 38, 369–378 CrossRef CAS PubMed; (b) M. Fujita, Chem. Soc. Rev., 1998, 27, 417–425 RSC.
- D. L. Caulder and K. N. Raymond, Acc. Chem. Res., 1999, 32, 975–982 CrossRef CAS.
- (a) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed; (b) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed; (c) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853–907 CrossRef CAS PubMed; (d) P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502–518 CrossRef CAS.
- (a) S. Zarra, D. M. Wood, D. A. Roberts and J. R. Nitschke, Chem. Soc. Rev., 2015, 44, 419–432 RSC; (b) A. M. Castilla, W. J. Ramsay and J. R. Nitschke, Acc. Chem. Res., 2014, 47, 2063–2073 CrossRef CAS PubMed.
- (a) E. C. Constable, Chem. Soc. Rev., 2013, 42, 1637–1651 RSC; (b) E. C. Constable, Chem. Soc. Rev., 2007, 36, 246–253 RSC.
- (a) M. D. Ward, Chem. Commun., 2009, 4487–4499 RSC; (b) P. J. Steel and C. M. Fitchett, Coord. Chem. Rev., 2008, 252, 990–1006 CrossRef CAS PubMed; (c) P. Dydio and J. N. H. Reek, Chem. Sci., 2014, 5, 2135–2145 RSC.
- (a) M. Poyatos, J. A. Mata and E. Peris, Chem. Rev., 2009, 109, 3677–3707 CrossRef CAS PubMed; (b) J. A. Mata, M. Poyatos and E. Peris, Coord. Chem. Rev., 2007, 251, 841–859 CrossRef CAS PubMed.
- (a) F. E. Hahn, C. Radloff, T. Pape and A. Hepp, Organometallics, 2008, 27, 6408–6410 CrossRef CAS; (b) C. Radloff, F. E. Hahn, T. Pape and R. Fröhlich, Dalton Trans., 2009, 7215–7222 RSC; (c) C. Radloff, J. J. Weigand and F. E. Hahn, Dalton Trans., 2009, 9392–9394 RSC; (d) F. M. Conrady, R. Fröhlich, C. Schulte to Brinke, T. Pape and F. E. Hahn, J. Am. Chem. Soc., 2011, 133, 11496–11499 CrossRef CAS PubMed; (e) M. Schmidtendorf, T. Pape and F. E. Hahn, Angew. Chem., Int. Ed., 2012, 51, 2195–2198 CrossRef CAS PubMed; (f) M. Viciano, M. Sanau and E. Peris, Organometallics, 2007, 26, 6050–6054 CrossRef CAS.
- (a) F. E. Hahn, C. Radloff, T. Pape and A. Hepp, Chem. – Eur. J., 2008, 14, 10900–10904 CrossRef CAS PubMed; (b) C. Radloff, H. Y. Gong, C. Schulte to Brinke, T. Pape, V. M. Lynch, J. L. Sessler and F. E. Hahn, Chem. – Eur. J., 2010, 16, 13077–13081 CrossRef CAS PubMed; (c) A. Rit, T. Pape and F. E. Hahn, J. Am. Chem. Soc., 2010, 132, 4572–4573 CrossRef CAS PubMed; (d) A. Rit, T. Pape, A. Hepp and F. E. Hahn, Organometallics, 2011, 30, 334–347 CrossRef CAS; (e) D. H. Wang, B. G. Zhang, C. He, P. Y. Wu and C. Y. Duan, Chem. Commun., 2010, 46, 4728–4730 RSC; (f) C. Segarra, G. Guisado-Barrios, F. E. Hahn and E. Peris, Organometallics, 2014, 33, 5077–5080 CrossRef CAS; (g) N. Sinha, F. Roelfes, A. Hepp, C. Mejuto, E. Peris and F. E. Hahn, Organometallics, 2014, 33, 6898–6904 CrossRef CAS.
- (a) O. Guerret, S. Sole, H. Gornitzka, M. Teichert, G. Trinquier and G. Bertrand, J. Am. Chem. Soc., 1997, 119, 6668–6669 CrossRef CAS; (b) B. Karimi and P. F. Akhavan, Chem. Commun., 2011, 47, 7686–7688 RSC; (c) B. Karimi and P. F. Akhavan, Inorg. Chem., 2011, 50, 6063–6072 CrossRef CAS PubMed; (d) B. Karimi and P. F. Akhavan, Chem. Commun., 2009, 3750–3752 RSC; (e) A. J. Boydston and C. W. Bielawski, Dalton Trans., 2006, 4073–4077 RSC; (f) L. Mercs, A. Neels and M. Albrecht, Dalton Trans., 2008, 5570–5576 RSC; (g) L. Mercs, A. Neels, H. Stoeckli-Evans and M. Albrecht, Dalton Trans., 2009, 7168–7178 RSC; (h) C. Zhang, J. J. Wang, Y. Liu, H. Ma, X. L. Yang and H. B. Xu, Chem. – Eur. J., 2013, 19, 5004–5008 CrossRef CAS PubMed; (i) J. Choi, H. Y. Yang, H. J. Kim and S. U. Son, Angew. Chem., Int. Ed., 2010, 49, 7718–7722 CrossRef CAS PubMed; (j) S. Gonell, M. Poyatos and E. Peris, Chem. – Eur. J., 2014, 20, 5746–5751 CrossRef CAS PubMed.
- (a) R. H. Crabtree, Coord. Chem. Rev., 2013, 257, 755–766 CrossRef CAS PubMed; (b) G. Guisado-Barrios, J. Bouffard, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 4759–4762 CrossRef CAS PubMed; (c) K. F. Donnelly, A. Petronilho and M. Albrecht, Chem. Commun., 2013, 49, 1145–1159 RSC.
- P. Mathew, A. Neels and M. Albrecht, J. Am. Chem. Soc., 2008, 130, 13534–13535 CrossRef CAS PubMed.
- (a) M. T. Zamora, M. J. Ferguson and M. Cowie, Organometallics, 2012, 31, 5384–5395 CrossRef CAS; (b) R. Maity, M. van der Meer and B. Sarkar, Dalton Trans., 2015, 44, 46–49 RSC; (c) S. Hohloch, S. Kaiser, F. L. Duecker, A. Bolje, R. Maity, J. Kosmrlj and B. Sarkar, Dalton Trans., 2015, 44, 686–693 RSC; (d) R. Maity, S. Hohloch, C.-Y. Su, M. van der Meer and B. Sarkar, Chem. – Eur. J., 2014, 20, 9952–9961 CrossRef CAS PubMed; (e) E. C. Keske, O. V. Zenkina, R. Wang and C. M. Crudden, Organometallics, 2012, 31, 456–461 CrossRef CAS; (f) E. C. Keske, O. V. Zenkina, R. Wang and C. M. Crudden, Organometallics, 2012, 31, 6215–6221 CrossRef CAS; (g) J. Cai, X. Yang, K. Arumugam, C. W. Bielawski and J. L. Sessler, Organometallics, 2011, 30, 5033–5037 CrossRef CAS PubMed; (h) R. Maity, M. Van der Meer, S. Hohloch and A. Sarkar, Organometallics, 2015, 34, 3090–3096 CrossRef CAS; (i) D. I. Bezuidenhout, G. Kleinhans, G. Guisado-Barrios, D. C. Liles, G. Ung and G. Bertrand, Chem. Commun., 2014, 50, 2431–2433 RSC; (j) G. Guisado-Barrios, J. Bouffard, B. Donnadieu and G. Bertrand, Organometallics, 2011, 30, 6017–6021 CrossRef CAS PubMed.
- C. Mejuto, G. Guisado-Barrios and E. Peris, Organometallics, 2014, 33, 3205–3211 CrossRef CAS.
- (a) I. J. B. Lin and C. S. Vasam, Coord. Chem. Rev., 2007, 251, 642–670 CrossRef CAS PubMed; (b) C. E. Strasser, E. Stander-Grobler, O. Schuster, S. Cronje and H. G. Raubenheimer, Eur. J. Inorg. Chem., 2009, 1905–1912 CrossRef CAS PubMed; (c) I. J. B. Lin and C. S. Vasam, Comm. Inorg. Chem., 2004, 25, 75–129 CrossRef CAS PubMed.
- D. Canseco-Gonzalez, A. Petronilho, H. Mueller-Bunz, K. Ohmatsu, T. Ooi and M. Albrecht, J. Am. Chem. Soc., 2013, 135, 13193–13203 CrossRef CAS PubMed.
Footnotes |
| † We would like to dedicate this work to Prof. Ekkehardt Hahn on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures and full characterization of new compounds, including NMR spectra. Computational details. CCDC 1406599. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc05114b |
| This journal is © The Royal Society of Chemistry 2015 |