Bioorthogonal labelling of living bacteria using unnatural amino acids containing nitrones and a nitrone derivative of vancomycin†
Abstract
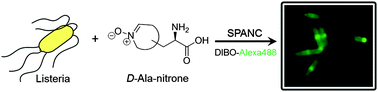
Unnatural D-amino acids bearing endocyclic nitrones were developed for live-cell labelling of the bacterial peptidoglycan layer. Metabolic incorporation of D-Lys and D-Ala derivatives bearing different endocyclic nitrones was observed in E. coli, L. innocua, and L. lactis. The incorporated nitrones of these bacteria then rapidly underwent strain-promoted alkyne–nitrone cycloaddition (SPANC) reactions affording chemically modified bacteria.

 Please wait while we load your content...
Please wait while we load your content...