Open Access Article
Open Access ArticleA facile solvent-free synthesis route for the assembly of a highly CO2 selective and H2S tolerant NiSIFSIX metal–organic framework†
Osama
Shekhah
,
Youssef
Belmabkhout
,
Karim
Adil
,
Prashant M.
Bhatt
,
Amy J.
Cairns
and
Mohamed
Eddaoudi
*
Functional Material Design, Discovery and Development (FMD3), Advanced Membrane & Porous Materials Center, 4700 King Abdullah University of Science and Technology, Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: mohamed.eddaoudi@kaust.edu.sa
First published on 6th July 2015
Abstract
The development of materials for CO2 capture with high selectivity and high tolerance to H2S is of prime importance for various industrially relevant gas streams (e.g. natural gas and biogas upgrading as well as pre-combustion capture). Here, we report the successful fabrication of a MOF with combined exceptional CO2 capture properties and H2S tolerance, namely the NiSIFSIX-based MOF using both solvothermal and solvent-free methodologies.
Metal–organic frameworks (MOFs), a special class of solid-state materials, have been considered to be one of the most burgeoning porous materials in the last two decades.1 MOFs are very attractive due to their modular nature, e.g. large porosity, chemical and topological versatility and structural diversity, which offer potential in various applications such as catalysis, gas storage, gas sensing, greenhouse gas emission mitigation and many other challenging gas separations.2,3
Recently, MOFs were intensively investigated for CO2 capture from various gas streams such as post-combustion, pre-combustion capture, natural gas and biogas upgrading.4 In spite of the large number of studies, only few reports focused on traces or low CO2 concentration removal applications (i.e. CO2 removal from air, hydrogen purification, and medical applications such as anaesthesia machines).5–7 Furthermore, development of materials capable of selective CO2 removal, in CO2 diluted streams, with high tolerance to H2S is of prime practical importance.
Our quest for made-to-order materials that can address efficiently the separation and capture of CO2 at different concentrations has prompted us to explore the potential of various MOFs with different structural properties and chemical compositions.4d,7–9
Recently, the successful practice of reticular chemistry allowed us to fabricate a series of isoreticular very stable MOFs with periodically arrayed hexafluorosilicate (SIFSIX) pillars, called SIFSIX-3-Zn4d and SIFSIX-3-Cu.7
These aforementioned porous MOFs disclose unique features for carbon capture, namely uniformly aligned strong CO2 adsorption sites in an idealized and contracted pore system with high localized charge density. Particularly the use of a relatively small cation, i.e. Cu2+, led to an isoreticular analogue with a very high selectivity toward CO2 adsorption at very low CO2 partial pressures when compared to other physical adsorbents reported in the open literature.4d,7
These unique materials offer great potential for air-capture applications due to the periodically aligned very high (non-reactive) CO2 adsorption energetic sites, resulting in a fully reversible physically driven adsorption–desorption operation under very mild conditions. The contracted pore system associated with a localized high charge density had afforded the selective CO2 adsorption with relatively much faster rates and stronger interactions than H2, CH4 and N2, and subsequently the attainment of unprecedented separation performances based on a unique synergistic mechanism involving both thermodynamics and kinetics in a wide range of CO2 concentrations.
It is worth noting that commonly hybrid porous solid-state materials are synthesized using solvo/hydrothermal routes.10,11 Various other synthetic approaches have been explored and reported for the construction of MOF materials such as (i) microwave-assisted solvothermal methods,11 (ii) micro-fluidics,11c (iii) ionic liquids,11d and (iv) electro-chemistry.11e Nevertheless, all these synthetic practices are found to be practical for the lab-scale research but are hardly implemented, and not often economically applicable, at the pilot or larger industrial scales. Mainly, the scale-up of MOF materials is hindered by the expensive cost, safety and the negative environmental impact associated with the use of organic solvents. Furthermore, MOF solution-based synthesis approaches suffer from additional drawbacks such as the plausible toxic and/or corrosive character associated with dissolved metal salts, generating acids as byproducts and often susceptible to solvolysis and must be disposed.11 Additionally, the need to dispose or recycle large quantities of solvents is not cost effective. All the above mentioned downfalls have hindered the timely progress in the scale-up of MOFs and their successful deployment in industrial applications.12 Accordingly, the successful use of the solvent-free synthesis route for the construction of MOFs can be regarded as a plausible route toward the requisite cost-effective scale-up of MOFs, a critical step for MOF industrial deployment.11a
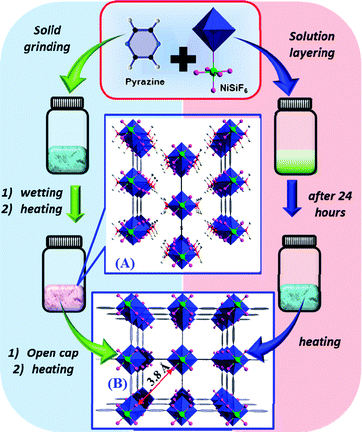
Here, we report the solvent-free synthesis of a new H2S tolerant MOF material, namely SIFSIX-3-Ni, isostructural to the Zn and Cu based SIFSIX-3-M,4d–7 with very high CO2 selectivity in a wide range of CO2 concentrations. After countless failed attempts to develop solvent-free procedures for the synthesis of the previously isolated Zn and Cu SIFSIX analogues (SIFSIX-3-Zn and SIFSIX-3-Cu),4d–7 we extended our focus and efforts to other metal sources that can also potentially form SIFSIX-3-M isostructures. Accordingly, first we opted to target and confirm the construction of the nickel analogue using the conventional solvothermal route and then subsequently explore the ability to fabricate this Ni-analogue using a free-solvent process.
Indeed, the SIFSIX-3-Ni analogue (B in Scheme 1) was prepared successfully by layering a methanol solution of pyrazine (pyz) in a glass tube onto a methanol solution of NiSiF6·xH2O. Upon layering, a slow formation of light blue powder was observed, and the powder was left for 24 hours in the mother solution. Before the gas adsorption study, the powder was first collected, washed extensively with methanol and then heated to 105 °C, dried under vacuum and characterized using powder X-ray diffraction (PXRD) (Fig. S1, ESI†). The structure of this expected new analogue is in very good agreement with those of the Zn and Cu analogues reported previously,4d,7 but with a slightly smaller unit cell (378 Å3) than the Zn (388 Å3) analogue which is attributed to the relatively strong bonding between the Ni2+ and the pyrazine as compared to Zn2+ (Table S1, ESI†).7,8 The unit cell size of the Ni analogue was found to be between the Zn and the Cu analogues, in good agreement with the relatively sharp pore size distribution (PSD) centred at 3.8 Å (average pore size), as determined from the CO2 adsorption isotherm analysis using the NLDFT model (Fig. 1b). This result is in good agreement with the associated crystal structure and as anticipated is situated between the observed values for SIFSIX-3-Zn (3.8–4 Å) and SIFSIX-3-Cu (3.5 Å).
 | ||
| Fig. 1 (a) Variable temperature adsorption isotherms of CO2 for SIFSIX-3-Ni, (b) pore size distribution from CO2 sorption isotherms at 77 K for SIFSIX-3-Ni. | ||
The steepness of variable temperature adsorption isotherms at low pressure for the newly isolated Ni analogue (Fig. 1a) is another indication of the relatively small pore size as compared to the Zn analogue. The intermediate steepness of CO2 adsorption isotherms is also an indication of stronger/weaker CO2–SIFSIX-3-Ni interactions as compared to the Zn/Cu analogues. Accordingly, the Qst of CO2 adsorption for SIFSIX-3-Ni (Fig. 2) is 7% and 8% higher/lower (48 kJ mol−1) than the Zn and the Cu analogues, respectively. The Qst of CO2 adsorption is an intrinsic property that dictates the affinity of the MOF toward CO2, which in turn plays an important role in determining the necessary energy to release CO2 during the regeneration step. The Qst for CO2 is in the range of fully reversible CO2 adsorption (30–50 kJ mol−1),4d in agreement with the full evacuation of SIFSIX-3-Ni at 298–323 K in a vacuum. As in the case of SIFSIX-3-Zn and SIFSIX-3-Cu, the Qst for CO2 adsorption was relatively constant indicating homogenous energetic sites over the full range of CO2 loading (Fig. 2).4d,7 By analogy and based on the full mixed gas adsorption and kinetics study carried out on the Zn and Cu analogues,4d,7 it is logically expected that SIFSIX-3-Ni will perform at least similarly as the Zn analogue4d in terms of CO2 separation from N2, O2, CH4 and H2 containing mixtures. The anticipated high selectivity toward CO2 in the case of SIFSIX-3-Ni is projected to be driven by the unique optimal combination between thermodynamics and kinetics, i.e., the occurrence of strong/homogenous CO2–MOF interactions and the relatively fast CO2 adsorption than those of other relevant gases.4d,7
The successful isolation of the new Ni analogue solvothermally had encouraged us to explore its plausible isolation using a solvent-free route. After few attempts, and in contrast to the case of Zn and Cu analogues, we successfully discovered the appropriate conditions that permit a solvent-free route synthesis of SIFSIX-3-Ni. This extremely facile methodology is based first on mechanically mixing both the organic and inorganic solid precursors i.e. pyrazine and NiSiF6 with a molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively, followed by a wetting procedure with few drops of water and then a careful heating procedure (Scheme 1).
1 respectively, followed by a wetting procedure with few drops of water and then a careful heating procedure (Scheme 1).
Indeed, after dry mechanical mixing and wetting, the examination of the resultant powder mixture using powder X-ray diffraction data showed that a very less crystalline product was obtained (Fig. 3a). The resultant powder mixture was heated to 65 °C for 4 hours in a closed vial which resulted in a colour change to light violet. The observed colour change is associated with the formation of the 2D structure of the SIFSIX-3-Ni analogue (A in Scheme 1). Interestingly, upon heating (drying) at 105 °C for an additional 4 hours, we observed a structural phase transformation. The PXRD diagram of the final material prepared using the solvent-free route method after the drying process was found to match and correspond to the 3D SIFSIX-3-Ni structure (B in Scheme 1) prepared using the solvothermal method (Fig. S1, ESI†). It is worth mentioning that the application of a similar solvent-free procedure to the Zn and Cu analogues did not permit the observation of the same phase transformation from the 2D to the 3D structure.
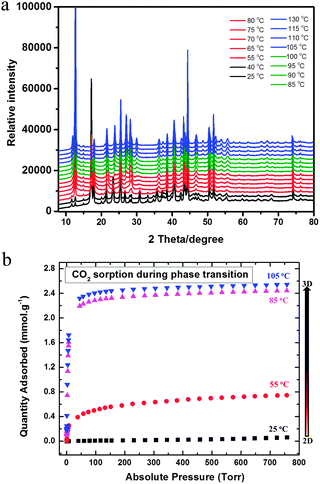 | ||
| Fig. 3 (a) Variable temperature PXRD for phase transition of the 2D (NiSiF6·xH2O) to the 3D SIFSIX-3-Ni structure, and (b) CO2 sorption at 298 K during phase transition at different temperatures. | ||
Essentially, the Rietveld refinement allowed us to clearly confirm the crystal structure of the resultant crystalline material from the solvent-free synthesis as SIFSIX-3-Ni (Fig. S1, ESI†). In order to better understand the crystallisation process affording the formation of SIFSIX-3-Ni, variable temperature PXRD was conducted (Fig. 3a) from 25 °C to 130 °C. At low temperature (25–40 °C), a first product with a very low crystallinity appeared which corresponds to a 2D structural analogue that was previously reported (CuSiF6(pyz)(H2O)2) (A in Scheme 1).7 A further increase in the temperature resulted in a phase transformation that afforded the formation of targeted 3D SIFSIX-3-Ni starting at around 55 °C and completed at 70 °C. At higher temperatures, PXRDs confirm the good crystallinity of the 3D material and its associated noticeable stability up to 130 °C.
It is worth noting that the phase transformation was accompanied by a discernible colour change from light violet to light blue when full converted.
Delightfully, the Ni analogue prepared by the solvent-free route showed the same variable temperature adsorption isotherms at low pressure as the Ni analogue prepared by the solvothermal methods which further supports the conformity of the SIFSIX-3-Ni materials resulting from both approaches (Fig. 3b). Fig. 3b illustrates the evolution in the CO2 adsorption uptake, recorded at 25 °C, as a function of the evacuation temperature for the SIFSIX-3-Ni analogue prepared by the solvent-free route method. The CO2 adsorption showed an increase in the CO2 uptake with increased evacuation temperatures up to 105 °C, corresponding to the optimal CO2 adsorption isotherm which also correlates with the phase change observed in the VT-PXRD and the complete conversion to 3D SIFSIX-3-Ni. The Qst for CO2 was found to be in good agreement with the associated values for SIFSIX-3-Ni prepared using the solvothermal method (Fig. S3, ESI†).
In light of the exceptional CO2 capture properties of SIFSIX-3-Zn and SIFSIX-3-Cu4d,7 analogues, it is expected that the high CO2 selectivity of SIFSIX-3-Ni at intermediate and high CO2 concentrations will be similarly high. For the particular case of SIFSIX-3-Ni we found that the structure is stable up to 80% RH both in CO2-humidity and CO2-air and undergo phase change to the 2D structure (Scheme 1A) at relative humidity higher than 80% RH (Fig. S2, ESI†).
In addition to favourable adsorption properties for CO2 capture, an ideal CO2 adsorbent should also be tolerant to impurities such as H2S, since natural gas contains acidic gases in various amounts that can reach high concentration up to 10–20% in the case of H2S. Accordingly, we have investigated the stability of SIFSIX-3-Ni after exposure and adsorption of H2S. It is worth noting that MOFs known to be stable upon exposure to H2S are scarce.13
The H2S sorption isotherm was collected for the SIFSIX-3-Ni sample and revealed an uptake of H2S around 1.3 mol g−1 at 10% concentration (Fig. 4). The H2S adsorption isotherm was repeated on the same sample in order to check/confirm the material's stability; the second H2S adsorption isotherm was found to be identical to the first one as shown in Fig. S4 (ESI†), and thus confirming the stability and regeneration/recycling of SIFSIX-3-Ni in the presence of H2S. This unique stability of the SIFSIX-3-Ni material in the presence of H2S was also supported by the similarities between the PXRD patterns before and after the H2S exposure (Fig. S2, ESI†). The resultant H2S exposed SIFSIX-3-Ni sample was then used for another cycle of CO2 sorption isotherm studies. Comfortably, the new collected CO2 adsorption isotherm matched exactly with the adsorption isotherm for the fresh sample not exposed to H2S as shown in Fig. S5 (ESI†). Certainly, the repeated H2S exposure did not affect/alter the subsequent capacity of SIFSIX-3-Ni for CO2 and H2S. It is worth stating that the Cu and Zn analogues are not tolerant to H2S, unlike the H2S tolerant SIFSIX-3-Ni, and lose their crystallinity and CO2 uptake capacity upon exposure to H2S. This further supports the uniqueness of SIFSIX-3-Ni and its prospective use as an excellent adsorbent for CO2 removal from a gaseous mixture containing acidic gases for natural gas upgrading application.
 | ||
| Fig. 4 CO2 and H2S sorption isotherms for SIFSIX-3-Ni. H2S uptake remains same after repeated sorption and activation cycles. | ||
In conclusion, we showed herein that the successful practice of MOF chemistry had permitted for the first time the fabrication of an H2S-tolerant MOF, SIFSIX-3-Ni, for practical CO2 capture in a wide range of CO2 concentrations, using a solvent-free synthetic approach. The exceptional CO2 capture and high tolerance to H2S, in addition to the simplified synthesis procedure, qualify the SIFSIX-3-Ni analogue as a prospective candidate for scale-up and testing at the pilot scale. This projected accomplishment will facilitate the smooth transition to commercialization and will pave the way for this technology technically and economically to be viable.
Research reported in this publication was supported by the King Abdullah University of Science and Technology (KAUST).
Notes and references
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- R. J. Kuppler, D. J. Timmons, Q. R. Fang, J. R. Li, T. A. Makal, M. D. Young, D. Q. Yuan, D. Zhao, W. J. Zhuang and H.-C. Zhou, Coord. Chem. Rev., 2009, 253, 3042–3066 CrossRef CAS PubMed.
- M. Shah, M. C. McCarthy, S. Sachdeva, A. K. Lee and H.-K. Jeong, Ind. Eng. Chem. Res., 2012, 51, 2179–2199 CrossRef CAS.
- (a) S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed; (b) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed; (c) A. Sayari, Y. Belmabkhout and R. Serna-Guerrero, Chem. Eng. J., 2011, 171, 760–774 CrossRef CAS PubMed; (d) P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80 CrossRef CAS PubMed.
- S. Choi, T. Watanabe, T.-H. Bae, D. S. Sholl and C. W. Jones, J. Phys. Chem. Lett., 2012, 3, 1136–1141 CrossRef CAS.
- T. M. McDonald, W. Ram Lee, J. A. Mason, B. M. Wiers, C. Seop Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef CAS PubMed.
- O. Shekhah, Y. Belmabkhout, Z. Chen, V. Guillerm, A. Cairns, K. Adil and M. Eddaoudi, Nat. Commun., 2014, 5, 4228 CAS.
- R. Luebke, J. F. Eubank, A. J. Cairns, Y. Belmabkhout, L. Wojtas and M. Eddaoudi, Chem. Commun., 2012, 48, 1455–1458 RSC.
- D.-X. Xue, A. J. Cairns, Y. Belmabkhout, L. Wojtas, Y. Liu, M. H. Alkordi and M. Eddaoudi, J. Am. Chem. Soc., 2013, 135, 7660–7667 CrossRef CAS PubMed.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer Jr., R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC.
- (a) A. Pichon, A. Lazuen-Garay and S. L. James, CrystEngComm, 2006, 8, 211 RSC; (b) T. Friščić, Chem. Soc. Rev., 2012, 41, 3493–3510 RSC; (c) M. Klimakow, P. Klobes, A. F. Thünemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216–5221 CrossRef CAS; (d) W. Yuan, J. O'Connor and S. L. James, CrystEngComm, 2010, 12, 3515–3517 RSC; (e) K. Fujii, A. Lazuen Garay, J. Hill, E. Sbircea, Z. Pan, M. Xu, D. C. Apperley, S. L. James and K. D. M. Harris, Chem. Commun., 2010, 46, 7572–7574 RSC; (f) G. A. V. Martins, P. J. Byrne, P. Allan, S. J. Teat, A. M. Z. Slawin, Y. Li and R. E. Morris, Dalton Trans., 2010, 39, 1758–1762 RSC; (g) H. Sakamoto, R. Matsuda and S. Kitagawa, Dalton Trans., 2012, 41, 3956–3961 RSC; (h) C. J. Adams, M. F. Haddow, M. Lusi and A. G. Orpen, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 16033–16038 CrossRef CAS PubMed.
- (a) I. A. Ibarra, P. A. Bayliss, E. Pérez, S. Yang, A. J. Blake, H. Nowell, D. R. Allan, M. Polyakoff and M. Schröder, Green Chem., 2012, 14, 117–122 RSC; (b) N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed; (c) R. A. Smaldone, R. S. Forgan, H. Furukawa, J. J. Gassensmith, A. M. Z. Slawin, O. M. Yaghi and J. F. Stoddart, Angew. Chem., Int. Ed., 2010, 49, 8630–8634 CrossRef CAS PubMed.
- L. Hamon, C. Serre, T. Devic, T. Loiseau, F. Millange, G. Férey and G. De Weireld, J. Am. Chem. Soc., 2009, 131, 8775–8777 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Structural characterization, H2S stability and adsorption experiments. CCDC 1404097. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc04487a |
| This journal is © The Royal Society of Chemistry 2015 |