 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A bi-stable Pt(II) based molecular turnstile†
Nicolas
Zigon
and
Mir Wais
Hosseini
*
Molecular Tectonics Laboratory, UMR UDS-CNRS, 7140 & icFRC, Université de Strasbourg, F-67000, Strasbourg, France. E-mail: hosseini@unistra.fr; Fax: +33 368851325; Tel: +33 368851323
First published on 26th June 2015
Abstract
Playing with the competition between H- and coordination-bonds, a bi-stable unsymmetrical organometallic turnstile was designed and its dynamic behaviour was studied in solution using NMR techniques. The turnstile, bearing two differentiated interaction sites, is based on a stator, composed of a square planar Pt(II) centre equipped with one pyridyl group and one phenol moiety, connected to a symmetrical rotor bearing a pyridyl unit as either a H-bond acceptor or as a monodentate coordinating site. Whereas in the absence of a metallic effector, the turnstile is locked by a H-bond formed between the phenol moiety located on the stator and the pyridyl group of the rotor, in the presence of Ag+ cations, the turnstile switches to another closed state resulting from the simultaneous binding of the cations by pyridyl units belonging to both the stator and the rotor. The switching process was shown to be reversible.
Controlling translational or rotational intramolecular movements within abiotic molecular entities is a topic of current interest.1 Among many systems reported, a class of molecules called molecular turnstiles2 is of particular interest for the design of bi-stable dynamic entities displaying an open and a closed state with the possibility of switching between the two by an external stimuli.
For some time now, our group has been investigating the design and synthesis of molecular turnstiles. Thus, we have reported a series of porphyrin based dynamic systems.3 We have also extended our approach to organic turnstiles with optical reading of their open and closed states.4 Finally, inspired by contributions on molecular gyroscopes by Gladysz et al.,5 we have reported a series of organometallic turnstiles based on Pt(II).6 Molecular turnstiles reported so far are dynamic entities based on open and closed states. To the best of our knowledge, no example of turnstiles undergoing a switch between two closed states has been reported.
Herein we report the design, synthesis and solution study of a Pt(II)-based organometallic turnstile and the switching between its two different closed states based either on H-bonding or coordination process (Fig. 1).
 | ||
| Fig. 1 The two closed states of the unsymmetrical turnstile 1 (C1) and its silver complex 1-Ag+ (C2). | ||
The design of the turnstile 1 (Scheme 1) is based on a square planar Pt(II) centre as a hinge, bearing two different sites, a phenol as a H-bond donor and a pyridyl moiety as a monodentate coordinating site. This ensemble may be regarded as the stator. The latter is equipped with a symmetric rotor also bearing a pyridyl group as either a H-bond acceptor site or as a monodentate coordinating group. The connection of the two sites located on the Pt(II) based stator is ensured by Pt–C bonds using two alkynyl units and the position 4 of both phenol and pyridine moieties. The rotor is connected to the stator by two Pt–P bonds using an α,ω-bis-diphenylphosphine fragment bearing a pyridyl unit at its centre. The interconnection between the pyridyl moiety and the two phosphine groups is ensured by two tetraethyleneglycol spacers using positions 2 and 6 of the aromatic group. The choice of stator and rotor terminology is arbitrary and may be reversed.
In principle, in the absence of a metal centre as the effector, the turnstile 1 should be in its closed state C1 resulting from the establishment of a H-bond between the phenol group of the stator and the pyridyl moiety belonging to the rotor. Upon the addition of an effector such as Ag+ cations, owing to the higher stability constant of the silver complex when compared to the H-bonded state, the turnstile should switch to a second closed state C2 based on the simultaneous binding of the cations by both pyridyl moieties belonging to the stator and the rotor (Fig. 1).
The synthesis (see ESI†) of the unsymmetrical turnstile 1 appeared to be rather challenging and required the exploration of three different synthetic strategies. The first one, statistical in nature, was based on the condensation at 55 °C of the metallamacrocycle 46a with the ethynylpyridine 57 and the protected ethynylphenol derivative 68 (1.5 eq. of each) in the presence of a catalytic amount of CuI in a THF/NEt3 mixture. As expected, the reaction afforded all three macrocyclic compounds: the desired unsymmetrical compound 1, and the two symmetrical turnstiles 26a and 3. Unfortunately, their separation was found to be unfeasible. However, upon deprotection of the phenol moiety using TetraButylAmmonium Fluoride (TBAF) at room temperature, the separation, although very tedious, could be achieved affording thus the desired turnstile 1 in 3.5% yield.
The second strategy, more directed, was based on the condensation of CODPtMeCl9 with the diphenylphosphine compound 76a under high dilution conditions. The reaction produced the intermediate 8 in 92% yield. The condensation of the latter with ethynyl pyridine 57 afforded the compound 9 with ca. 5–10% of the symmetrical bispyridyl turnstile 2. After substitution of the methyl group by chloride using HCl leading to the compound 10, the latter was condensed with the protected phenol 6.8 Again, the purification appeared to be very tedious. In order to overcome this, the TBDMS protective group was removed. After column chromatography and purifying several times by PTLC, the desired turnstile 1 was obtained in 6% yield (over the three last synthetic steps).
Finally, a third synthetic strategy was explored. This was based on mono-alkylation of the Pt(II)-metallamacrocycle 4 upon its reaction with stannylated 4-ethynylpyridine, generated in situ upon successive additions of nBuLi and SnMe3Cl.10 The condensation afforded compound 10. The reaction of the latter with 6 afforded the protected intermediate metallamacrocycle 11 which, upon deprotection using TBAF, afforded the desired turnstile 1 in 13% yield over the last three synthetic steps. The rather low yield reported for 1 corresponds to the isolated compound and results from the purification method, mainly, chromatography which appeared to be the only possible one.
The switching between the two C1 and C2 closed states of the unsymmetrical turnstile 1 (Fig. 1) was studied in CD2Cl2 solution using 1- and 2-D NMR techniques. All the H-atoms of 1 were assigned (Scheme 1).
For the turnstile 1, chemical shifts (ppm) Ht and Hu of the phenol moiety and HT and HU of the pyridyl group appeared at 6.95, 6.62 and 6.23, 8.09 respectively. For the phenol group, the chemical shifts observed are downfield shifted when compared to signals for the symmetrical bis-phenol turnstile 3 (Ht 6.60 and Hu 6.51). The chemical shifts for HT and HU of the pyridyl moiety of 1 are close to those observed for the unbound pyridyl group of 2-Ag+ at low temperature.6a Finally, the chemical shifts observed for Ha (7.75) and Hb (7.37) atoms of the pyridyl group located on the rotor in 1 are similar to those observed for the bis-phenol turnstile 3. These observations indicate the formation of a H-bond between the phenol moiety of the stator (H bond donor) and the pyridyl group of the rotor (H bond acceptor) leading thus to the peripheral positioning of the pyridyl unit of the stator with respect to the macrocyclic cavity.
Observations obtained by 1D 1H-NMR investigations mentioned above were further confirmed by 1H–1H ROESY NMR experiments (Fig. 2). The chemical shift of the signals in the regions corresponding to the aromatic and ethylene glycol H atoms shows cross-relaxation peaks between Ht and Hu of the phenol moiety and Hd–j–k–l of the handle. As expected, no cross-relaxation peaks between HT and HU of the pyridyl moiety of the stator and Hd–j–k–l atoms of the handle were observed. This indeed confirms that the turnstile 1 is locked in the closed state C1.
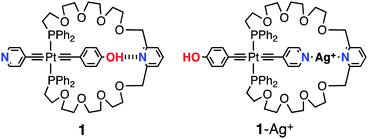
 | ||
| Fig. 2 Portion of the 1H–1H ROESY spectra of 1 (CD2Cl2, 500 MHz, 298 K). For signal assignments, see Scheme 1. | ||
The switch between the two closed states C1 and C2 was achieved upon addition of Ag+ cations as an effector. Indeed, addition of one equivalent of AgSbF6 in CD3CN to a solution of 1 in CD2Cl2, caused significant changes in the 1H NMR spectra of the turnstile, especially in the aromatic region (Fig. 3a and b, left). Hydrogen atoms of the stator are all strongly affected, with shifts (ppm) of −0.46, −0.23 and +0.36 ppm for Ht, Hu and HU, respectively. It should be noticed that the signal corresponding to HT is hidden under the Ho–p signals. This is in agreement with the previous observation of the signal at 7.5 ppm for 2-Ag+ at a low temperature.6a Furthermore, the signal corresponding to Ha is downfield shifted (δ = 7.94 ppm) upon binding of Ag+ cations. Again, this is in agreement with previous observation for 2-Ag+ for which the signal corresponding to Ha appears at 7.96 ppm.6a These observations imply the simultaneous binding of the silver cations by both pyridyl units belonging to the stator and the rotor.
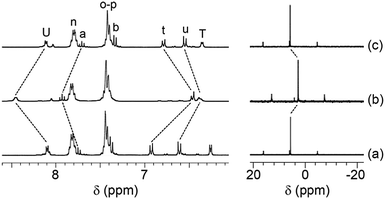 | ||
| Fig. 3 Portions of the 1H-NMR (left) and 31P-NMR (right) (CD2Cl2, 298 K) spectra for 1 (a), 1-Ag+ (b) and 1-Ag+ after the addition of one equivalent of Br− anions (c). For signal assignments, see Scheme 1. | ||
Again, the above-mentioned conclusions are further confirmed by 2D NOESY experiments. Indeed, the 1H–1H NOESY spectrum (Fig. 4) reveals the absence of correlations between the phenolic hydrogen atoms Ht and Hu of the stator and hydrogen atoms of the handle. This indicates that the phenol moiety is located outside of the macrocyclic backbone. Furthermore, as expected, HU correlates with Hf.
 | ||
| Fig. 4 Portion of the 1H–1H NOESY spectrum for 1-Ag+ (CD2Cl2, 500 MHz, 298 K). For signal assignments, see Scheme 1. | ||
The stoichiometry of the binding of silver cations by the turnstile 1 was investigated by mass spectrometry by addition of increasing amounts of AgSbF6, to a CH2Cl2 solution of 1 (see ESI†). For the 1/1 ratio of Ag+ and 1, the main product obtained is 1-Ag+. Upon increasing the ratio to 2 or 3, no trace of dicationic species was present; however, degradation of the complex was observed, which was not further investigated.
The reversibility of the switching process between the two closed states C1 and C2 was achieved upon the addition of one equivalent of Et4NBr to a solution of 1-Ag+ followed by the filtration of the precipitated AgBr salt. The process was monitored by both 1H- and 31P-NMR studies (Fig. 3). Indeed, chemical shifts for signals corresponding to Ht, Hu, HT, HU, Ha and Hb atoms were identical to those observed for 1 (Fig. 3, left). The same behaviour was observed when monitoring the process by 31P-NMR (Fig. 3, right).
The solution dynamic behaviour of the symmetrical bis-phenol turnstile 3 was also studied by 1- and 2-D NMR. In that case, as for the unsymmetrical turnstile 1, one would expect a closed state resulting from the formation of a H-bond between one of the two phenol units of the stator and the pyridyl group of the rotor. However, as previously observed for the other symmetrical turnstile 2 in the presence of silver cations,6a owing to the rather weak nature of the H bond, one would expect an oscillation between two equivalent closed states. At room temperature in CD2Cl2, the two phenol groups were found to be equivalent as only a single set of signals was observed for the Ht and Hu atoms (Fig. 5). This implies either the absence of the H-bond and thus the open state of the turnstile 3 or a rapid exchange between two equivalent closed states resulting from the formation of a H bond. In order to investigate the exchange process, variable temperature NMR studies have been carried out. Unfortunately, lowering the temperature to −46 °C did not allow the observation of the splitting of H atoms belonging to the two phenol groups into Ht and Hu and Ht′ and Hu′ as one would expect for the phenol involved in the H bond formation and the free phenol respectively. In order to enhance the strength of the H-bond, the 1H-NMR spectrum was recorded in a less polar mixture of solvents (CD2Cl2/CCl4 1/1). Unfortunately, the turnstile 3 appeared to be insoluble below −20 °C. The existence of the closed state of the turnstile 3 was demonstrated by 2D 1H–1H NOESY NMR that clearly revealed the presence of correlations peaks between the Ht and Hu atoms of the phenol ring and the H atoms of –OCH2– groups of the rotor (Fig. 5).
 | ||
| Fig. 5 Portion of the 1H–1H ROESY spectra (CD2Cl2, 600 MHz, 298 K) for the turnstile 3. For signal assignments, see Scheme 1. | ||
In conclusion, the unsymmetrical organometallic turnstile 1, based on a Pt(II) centre bearing a phenol and a pyridyl and connected to a symmetrical handle, displays two distinct closed states resulting either from the establishment of a H-bond between the phenol moiety and the pyridyl unit (C1) or the simultaneous binding of silver cations by both pyridyl units belonging to the stator and the rotor (C2). The switching between the two states is reversible and may be achieved by adding Br− anions, which leads to the precipitation of the insoluble AgBr salt. Based on the principle described in this contribution, the design of other organometallic turnstiles and metallic effectors is currently under investigation (Fig. 6).
This contribution was financially supported by the University of Strasbourg, the International Centre for Frontier Research in Chemistry (icFRC), Strasbourg, the Institut Universitaire de France, the CNRS and the Ministry of Education and Research (PhD fellowship to N. Z.).
Notes and references
- (a) J.-P. Sauvage, Science, 2001, 291, 2105 CrossRef CAS; (b) J.-P. Sauvage, Acc. Chem. Res., 1998, 31, 611 CrossRef CAS; (c) J.-P. Sauvage, in Molecular machines and motors, structure and bonding, ed. J.-P. Sauvage, Springer, Berlin, Heidelberg, vol. 99, 2001, pp. 1–282 Search PubMed; (d) V. Balzani, M. Venturi and A. Credi, Molecular devices and machines: a journey into the nanoworld, Wiley-VCH, Weinheim, 2003, pp. 1–457 CrossRef PubMed; (e) T. R. Kelly, in Molecular machines, Topics in Current Chemistry, ed. T. R. Kelly, vol. 262, Springer, Berlin, Heidelberg, 2005, pp. 1–227 CrossRef; (f) Y. Shirai, J.-F. Morin, T. Sasaki, J. M. Guerrero and J. M. Tour, Chem. Soc. Rev., 2006, 35, 1043 RSC; (g) E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72 CrossRef CAS PubMed; (h) N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152 CrossRef CAS PubMed; (i) V. Balzani, A. Credi, F. M. Raymo and J. F. Stoddart, Angew. Chem., Int. Ed., 2000, 39, 3348 CrossRef CAS; (j) C. A. Schalley, K. Beizai and F. Vögtle, Acc. Chem. Res., 2001, 34, 465 CrossRef CAS PubMed; (k) J. Michl and E. C. H. Sykes, ACS Nano, 2009, 3, 1042 CrossRef CAS PubMed; (l) G. Vives and J. M. Tour, Acc. Chem. Res., 2009, 42, 473 CrossRef CAS PubMed; (m) N. Weibel, A. Mishchenko, T. Wandlowski, M. Neuburger, Y. Leroux and M. Mayor, Eur. J. Org. Chem., 2009, 6140 CrossRef CAS PubMed; (n) S. Sengupta, M. E. Ibele and A. Sen, Angew. Chem., Int. Ed., 2012, 51, 8434 CrossRef CAS PubMed; (o) C. Clavel, C. Romuald, E. Brabet and F. Coutrot, Chem. – Eur. J., 2013, 19, 2982 CrossRef CAS PubMed.
- (a) T. C. Bedard and J. S. Moore, J. Am. Chem. Soc., 1995, 117, 10662 CrossRef CAS; (b) A. Carella, J. Jaud, G. Rapenne and J.-P. Launay, Chem. Commun., 2003, 2434 RSC; (c) N. Weibel, A. Mishchenko, T. Wandlowski, M. Neuburger, Y. Leroux and M. Mayor, Eur. J. Org. Chem., 2009, 6140 CrossRef CAS PubMed.
- (a) A. Guenet, E. Graf, N. Kyritsakas, L. Allouche and M. W. Hosseini, Chem. Commun., 2007, 2935 RSC; (b) A. Guenet, E. Graf, N. Kyritsakas and M. W. Hosseini, Inorg. Chem., 2010, 49, 1872 CrossRef CAS PubMed; (c) A. Guenet, E. Graf, N. Kyritsakas and M. W. Hosseini, Chem. – Eur. J., 2011, 17, 6443 CrossRef CAS PubMed; (d) T. Lang, A. Guenet, E. Graf, N. Kyritsakas and M. W. Hosseini, Chem. Commun., 2010, 46, 3508 RSC; (e) T. Lang, E. Graf, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2011, 40, 3517 RSC; (f) T. Lang, E. Graf, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2011, 40, 5244 RSC; (g) T. Lang, E. Graf, N. Kyritsakas and M. W. Hosseini, Chem. – Eur. J., 2012, 18, 10419 CrossRef CAS PubMed; (h) T. Lang, E. Graf, N. Kyritsakas and M. W. Hosseini, New J. Chem., 2013, 37, 112 RSC.
- N. Zigon, P. Larpent, A. Jouaiti, N. Kyritsakas and M. W. Hosseini, Chem. Commun., 2014, 50, 5040 RSC.
- (a) A. J. Nawara, T. Shima, F. Hampel and J. A. Gladysz, J. Am. Chem. Soc., 2006, 128, 4962 CrossRef CAS PubMed; (b) K. Skopek, M. C. Hershberger and J. A. Gladysz, Coord. Chem. Rev., 2007, 251, 1723 CrossRef CAS PubMed; (c) T. Shima, E. B. Bauer, F. Hampel and J. A. Gladysz, Dalton Trans., 2004, 1012 RSC; (d) E. B. Bauer, F. Hampel and J. A. Gladysz, Organometallics, 2003, 22, 5567 CrossRef CAS; (e) P. D. Zeits, G. P. Rachiero, F. Hampel, J. H. Reibenspies and J. A. Gladysz, Organometallics, 2012, 31, 2854 CrossRef CAS.
- (a) N. Zigon, A. Guenet, E. Graf and M. W. Hosseini, Chem. Commun., 2013, 49, 3637 RSC; (b) N. Zigon, A. Guenet, E. Graf, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2013, 43, 9740 RSC; (c) N. Zigon, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2013, 43, 152 RSC.
- L. Yu and J. S. Lindsey, J. Org. Chem., 2001, 66, 7402 CrossRef CAS PubMed.
- (a) G. Y. Fang and V. K. Aggarwal, Angew. Chem., Int. Ed., 2007, 46, 359 CrossRef CAS PubMed; (b) T. Ogawa, K. Ohta, T. Iijima, T. Suzuki, S. Ohta and Y. Endo, Bioorg. Med. Chem., 2009, 17, 1109 CrossRef CAS PubMed; (c) S. K. Chitneni, C. M. Deroose, J. Balzarini, R. Gijsbers, S. Celen, Z. Debyser, L. Mortelmans, A. M. Verbruggen and G. M. Bormans, J. Med. Chem., 2007, 50, 6627 CrossRef CAS PubMed.
- G. P. C. M. Dekker, A. Buijs, C. J. Elsevier, K. Vrieze, P. W. N. M. Van Leeuwen, W. J. J. Smeets, A. L. Spek, Y. F. Wang and C. H. Stam, Organometallics, 1992, 11, 1937 CrossRef CAS.
- M. Hissler, A. Harriman, A. Khatyr and R. Ziessel, Chem. – Eur. J., 1999, 5, 3366 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, full characterisation of new compounds, NMR and mass spectra. See DOI: 10.1039/c5cc04456a |
| This journal is © The Royal Society of Chemistry 2015 |


