Open Access Article
Open Access ArticleThe reactions of TiCl3, and of UF4 with TiCl3 in liquid ammonia: unusual coordination spheres in [Ti(NH3)8]Cl3·6NH3 and [UF(NH3)8]Cl3·3.5NH3†
Patrick
Woidy
a,
Antti J.
Karttunen
b,
Stefan S.
Rudel
a and
Florian
Kraus
*a
aAnorganische und Fluorchemie der Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35032 Marburg, Germany. E-mail: florian.kraus@chemie.uni-marburg.de
bDepartment of Chemistry, Aalto University, FI-00076 Aalto, Finland
First published on 25th June 2015
Abstract
TiCl3 and NH3 form octaammine titanium(III) chloride ammonia (1/6), [Ti(NH3)8]Cl3·6NH3, which is the first structurally characterized octaammine complex of a transition metal. An excess of TiCl3 reacts with UF4 in liquid NH3 and forms octaammine fluorido uranium(IV) chloride ammonia (1/3.5), [UF(NH3)8]Cl3·3.5NH3. It shows a distorted threefold-capped trigonal-prismatic coordination sphere around U(IV).
In the recent decades uranium chemistry experienced a renaissance, as more “non-nuclear” uses of uranium compounds, for example, in small molecule activation,1 or catalysis were investigated.2,3 Also, the workup of radioactive wastes and actinoid containing nuclear fuels, the selective extraction of actinoids, as well as the understanding of their geological fate in the environment after an accident, requires a profound knowledge of actinoid species in solution.4 Single crystal X-ray structures would be of aid for the assessment of potential structural motifs in the solution chemistry of the actinoids. However, structural information from single crystal X-ray diffraction was often not available as olation and oxolation reactions occur with actinoids in aqueous solutions. These were believed to lead to “ill-defined structures and chemistry” and thus it remained crystallographically unexplored.4 The structural chemistry of actinoid complexes obtained from aqueous solutions is therefore rather poorly understood. Liquid ammonia is a solvent system similar to water,5 and it may serve as a model system to obtain crystalline actinoid species which are otherwise difficult or impossible to isolate from aqueous media. Liquid ammonia shows a much lower autoprotolysis and has a broader pH range which may allow easier access to products coming from protonation and deprotonation reactions. The literature on the coordination chemistry of uranium with only simple, inorganic, monodentate ligands, with the exception of the aqua ligand, is very scarce compared to the magnitude of literature of multidentate-binding organic ligands.6–8 Uranium compounds may feature various coordination numbers,8,9 often from six to eight, but also higher in the case of multidentate ligands, and even coordination numbers of twelve or more, for example in the borohydrides, are known.10–12 A coordination number of nine for monodentate ligands is, however, rare and has, to the best of our knowledge, been reported only for the threefold capped trigonal prismatic cations of Ba2+,13 La3+,14 Sm3+,15 trivalent actinoids47 and for uranium compounds with DMSO, DMF, or acetonitrile as ligands.16–19 Our compound seems to be a rare example of such a threefold-capped trigonal-prismatic coordination sphere where only simple inorganic ligands are present on the uranium atom. We have previously investigated the reactions of UF4 and UF6 with anhydrous ammonia.20 In our attempts to identify usable fluoride ion acceptors for the liquid ammonia system,20–22 and in order to expand the chemistry of UF4, we report herein the usage of titanium(III) chloride as a fluoride ion acceptor in anhydrous ammonia besides the unusual coordination spheres of U(IV) and Ti(III) in the resulting compounds.
The coordination chemistry of titanium has been extensively reviewed.23,24 Titanium(III)-complexes of the formula types TiL63+, TiX2L4+ or TiX63− show an octahedron-like coordination. In aqueous solution the hexaaqua-cation [Ti(H2O)6]3+ is known.25 In a reaction of TiI3 in liquid ammonia at room temperature, only a byproduct with the composition [(NH3)5Ti–O–Ti(NH3)5]I4·NH3 (octahedral coordination) was observed.26 A square-antiprismatic coordination sphere for titanium has been reported for multidentate ligands, such as bidentate binding oxalate,27 and tridentate binding C16H18N4,28 and in the Ti8Bi929 and TiMn2P12 (Ti(IV)) compounds.30 For monodentate ligands, a Ti atom was observed in a metal–organic-framework compound which was coordinated by seven H2O ligands and one hydroxide anion in a distorted square-antiprismatic manner.31 Octaammine complexes of other metal cations are also quite rare, and structural proof is only available in the case of the twofold capped trigonal prismatic cations [M(NH3)8]2+ (M = Ca, Sr, and Ba),32–34 and the square-antiprismatic cation [Yb(NH3)8]3+.14,35 During the synthesis of TiX3·6NH3 (X = Cl and Br) colorless-greyish compounds with the composition of TiBr3·8NH3 and TiCl3·7NH3 have been observed without the possibility of further characterization.36 Other investigations in the TiCl3/NH3 system also showed that colorless-greyish TiCl3·6NH3 was always obtained as the only product.37,38 In all these cases, the colorless appearing solids were allowed to warm to room temperature after removal of the excess liquid NH3 and the compositions were then determined by elemental analyses.
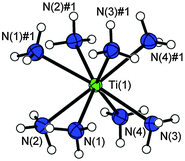
Pure TiCl3 reacts with anhydrous liquid ammonia at −40 °C to form colorless crystals of octaammine titanium(III) chloride ammonia(1/6), [Ti(NH3)8]Cl3·6NH3, the composition of which was elucidated using single-crystal X-ray analysis at low temperature (details available in Table S1, ESI†). The observed color is in agreement with reports on other Ti(III)–ammine complexes (see above) and pale blue colors may be easily overlooked as the moisture and temperature sensitive crystals had to be manipulated in dry, cold perfluoro-ether oil. The compound crystallizes in the monoclinic space group P2/c. The asymmetric unit contains a Ti(1) atom on the 2e position, which is coordinated by eight ammine ligands (N(1) to N(4) and symmetry equivalents) in the shape of a square antiprism (Fig. 1). Due to symmetry, the centers of both squares are 1.2619(7) Å away from the titanium atom and are tilted only by 1° towards each other. Hoffmann and coworkers analyzed the deviation from S8-symmetry by measuring the angle between the metal–ligand bond and the S8-axis.39 In an ideal system this angle should be 59.22°. Kepert, however, showed that the ideal S8-symmetry is slightly less stable compared to a marginally distorted arrangement of the ligands where the respective angle is 57.1°.40 In the titanium compound the angles are observed with 57.1° (Ti(1)–N(1)–S8-axis) and 56.2° (Ti(1)–N(2)–S8-axis) and thus the coordination polyhedron is best described as distorted square-antiprismatic. The Ti–N-distances are observed in between 2.275(1) and 2.312(1) Å and are therefore slightly larger compared to the ones of the [(NH3)5Ti–O–Ti(NH3)5]I4·NH3 compound, for which Ti–N distances of 2.21(1) to 2.29 Å were reported.26 This is of course due to the higher coordination number in [Ti(NH3)8]Cl3·6NH3. Other ammine complexes of the titanium group, for example M(NH3)F4 or [M(NH3)4F4]·NH3 (M = Zr and Hf), show similar M–N distances of 2.337(4), 2.29(2) Å, and 2.397(3), 2.383(8) Å, respectively.41,42 In the [Yb(NH3)8]3+ cation the Yb–N distances were reported in the range of 2.4 to 2.5 Å.14,35 Further selected atomic distances and angles of the crystal structure are available from Table S2 in the ESI† and from the caption of Fig. 1.
The chlorine atoms Cl(1) and Cl(2) occupy the crystallographic sites 4g and 2f, respectively. With a Ti⋯Cl-distance of 4.1374(4) and 4.7104(2) Å there is no direct cation–anion contact. The chloride anions act as acceptors for N–H⋯Cl–hydrogen bonds, for details see the ESI.† The unit cell of the [Ti(NH3)8]Cl3·6NH3 compound is shown in Fig. 2. It is interesting to note that the compound presented here shows a Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N-ratio of 1
N-ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, which has not been reported previously. Schläfer and coworkers report that TiCl3·6NH3 is obtained when ammonia is carefully pumped off from the reaction mixture of TiCl3 in liquid ammonia at –54 °C for five to six weeks,36 in our case we obtained the crystals of [Ti(NH3)8]Cl3·6NH3 under autogenous pressure at – 40 °C. As Schläfer and coworkers have obtained “only” TiCl3·6NH3,36 it is plausible to assume that [Ti(NH3)8]Cl3·6NH3 can be converted to yield the respective compound. The decomposition of TiCl3·6NH3 has been studied in the temperature range from −40 to +450 °C.43 At room temperature TiCl3·6NH337 as well as TiCl3·5NH3 were both reported to be stable.43 TiCl3·6NH3 decomposes to TiCl3·5NH3 at +7 °C.43 The crystals of [Ti(NH3)8]Cl3·6NH3 burst upon warming to temperatures higher than approximately −40 °C without autogenous pressure present. At room temperature TiCl3·5NH3 was obtained as evidenced by elemental analysis (calc.: N: 29.26%; H: 6.32%, det.: 29.83%, 6.231%).
14, which has not been reported previously. Schläfer and coworkers report that TiCl3·6NH3 is obtained when ammonia is carefully pumped off from the reaction mixture of TiCl3 in liquid ammonia at –54 °C for five to six weeks,36 in our case we obtained the crystals of [Ti(NH3)8]Cl3·6NH3 under autogenous pressure at – 40 °C. As Schläfer and coworkers have obtained “only” TiCl3·6NH3,36 it is plausible to assume that [Ti(NH3)8]Cl3·6NH3 can be converted to yield the respective compound. The decomposition of TiCl3·6NH3 has been studied in the temperature range from −40 to +450 °C.43 At room temperature TiCl3·6NH337 as well as TiCl3·5NH3 were both reported to be stable.43 TiCl3·6NH3 decomposes to TiCl3·5NH3 at +7 °C.43 The crystals of [Ti(NH3)8]Cl3·6NH3 burst upon warming to temperatures higher than approximately −40 °C without autogenous pressure present. At room temperature TiCl3·5NH3 was obtained as evidenced by elemental analysis (calc.: N: 29.26%; H: 6.32%, det.: 29.83%, 6.231%).
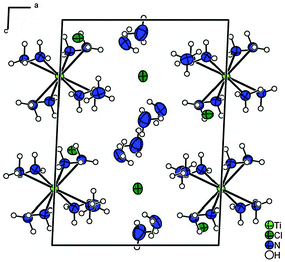 | ||
| Fig. 2 The unit cell of the [Ti(NH3)8]Cl3·6NH3 compound. Displacement ellipsoids are shown at the 70% probability level at 123 K, H-atoms isotropic with arbitrary radii. | ||
Aqueous solutions of Ti(III) are known to be violet/blue. Typically they show small extinction coefficients as the electronic d–d transition is forbidden. [Ti(NH3)8]3+ crystallizes in colorless crystals of [Ti(NH3)8]Cl3·6NH3, suggesting that it might show interesting electronic differences in comparison to the known Ti(III) compounds. Because the compound is not stable above ca. −40 °C, we could not obtain a UV/VIS spectrum. Instead, we investigated the spectroscopic properties of [Ti(NH3)8]3+ using ab initio quantum chemical methods high level coupled cluster calculations at the CC2/def2-TZVPP level of theory; for further computational details, see Experimental section. We first investigated the well-known Ti(III) complex [Ti(H2O)6]3+ as a reference case. A broad absorption band in the region around 500 nm has been reported in the most recent experimental UV/VIS spectrum, in agreement with computational results obtained with the DFT-B3LYP method.44 The UV/VIS results predicted here are in good agreement with the experiment, showing an absorption band centered at 490 nm. The transition strength is very low (0.2 × 10−6 a.u.), as expected for a forbidden d–d transition. In the case of the [Ti(NH3)8]3+ complex, the predicted absorption wavelength is noticeably red-shifted to 711 nm. Therefore, the [Ti(NH3)8]3+ complex should absorb in the red regime of the spectrum, suggesting a pale blue complementary color that might be rather difficult to observe properly, e.g. from crystals in cooled perfluoro-ether oil. Also for [Ti(NH3)8]3+, the transition strength of the absorption is very small (0.2 × 10−7 a.u.). A comparison between the frontier orbitals of [Ti(H2O)6]3+ and [Ti(NH3)8]3+ shows that in both cases the HOMO is the Ti dz2 orbital, which is also in line with the previous results for [Ti(H2O)6]3+.44 The other four d orbitals are ordered in two practically degenerate pairs. For [Ti(NH3)8]3+, the lower-lying pair of d-orbitals is composed of dx2−y2 and dxy, but the 711 nm absorption involves the higher-energy dxz and dyz orbitals. This energy ordering for the square antiprismatic [Ti(NH3)8]3+ ion is reversed in comparison to the octahedral [Ti(H2O)6]3+ complex.44 The square antiprismatic configuration of [Ti(NH3)8]3+ is non-centrosymmetric, but the dz2 → (dxz/dyz) transition is still symmetry-forbidden.
By comparing the radii/charge-ratios of Ti(III) and U(IV) for various coordination numbers (for C.N. 6: 0.14 and 0.17, respectively),45 and in view of our experiment Ti3+ should be a slightly harder Lewis-acid and therefore prefer the bonding to “harder” F−-anions over “softer” ammine ligands. When UF4 is reacted with TiCl3 in liquid ammonia solution, the reaction may be described by eqn (1):
| 2 UF4(am) + 2 TiCl3(am) + 23 NH3 → 2 [UF(NH3)8]Cl3·3.5NH3 + 2 “TiF3”(am) | (1) |
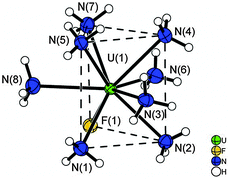
The identity of the resulting titanium(III) compound could not be established besides numerous attempts and is therefore referred to as “TiF3”. It is obtained in the form of a greyish precipitate of variable N, H, and F content, and is X-ray amorphous. Its IR spectrum shows that besides bound NH3 molecules, NH2− and possibly NH2− anions may also be present. The [UF(NH3)8]Cl3·3.5NH3 compound is obtained in the form of green crystals. The composition has been elucidated using single-crystal X-ray analysis (details in Table S1, ESI†). The atoms of the asymmetric unit of [UF(NH3)8]Cl3·3.5NH3 occupy only two crystallographic sites: the atoms U(1), F(1), N(1) to N(10), N(12), and Cl(1) to Cl(3) reside on the 8f position and the nitrogen atom N(11) occupies the 4e position. The uranium atom is coordinated by one fluorine atom and eight ammine ligands (N(1–8)) forming the octaammine fluorido uranium(IV) cation [UF(NH3)8]3+, shown in Fig. 3. The coordination polyhedron may be best described as a distorted, trifold-capped trigonal prism. The trigonal faces, which are formally formed by the atoms N(1), N(2), F(1) and N(4), N(5), N(7), respectively, are not parallel to each other but deviate by 5.24(8)°. The centers of these faces are 1.872(1) and 1.648(1) Å away from the U atom, respectively. The tetragonal planes show angles of 63.27(3), 58.37(4) and 58.38(4)° towards each other. They are capped by the ammine ligands N(6), N(8), and N(3) with distances of 1.755(2), 1.734(2), and 1.671(2) Å, to these planes, respectively. The nitrogen atoms of the tetragonal face deviate only by ±0.049(1) Å from the respective least-squares-planes.
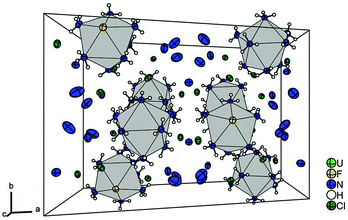
The U–N distances are observed in the range between 2.559(1) and 2.630(1) Å. They are comparable with distances reported for the [UF4(NH3)4]·NH3 compound with 2.618(5) Å,20 where the U-atom is only eightfold coordinated. Despite the coordination number of nine, the U–F-distance is quite short with 2.117(1) Å compared to the respective distances reported for the [UF4(NH3)4]·NH3 (2.188(4) Å) and (N2H7)(NH4)[UF7(NH3)] (2.200(2) to 2.336(2) Å) compounds, where the coordination number is only eight.20 For the [UO2F2(NH3)3]2·2NH3 compound longer U–F-distances ranging from 2.217(2) to 2.241(2) Å have been observed.46 So, the uranium(IV) cation seems to exert a strong pull towards the F− anion. Selected atomic distances and angles of the crystal structure are available from Table S4 (ESI†) and the caption of Fig. 3. The short N⋯Cl-, N⋯F- and N⋯N-distances are indicative of the presence of the respective hydrogen bonds, for details see the ESI.†Fig. 4 shows the unit cell of the compound. Upon warming to room temperature an X-ray amorphous powder is obtained, the IR spectrum of which shows the presence of ammine ligands. As the compound cannot be separated from the titanium compounds, elemental analysis was not undertaken. Our compound seems to be a rare example of a mononuclear uranium complex with monodentate ligands showing coordination number nine,4,47 and only a few other examples have been characterized, however with organic ligands such as dimethylformamide, acetonitrile, and dimethylsulfoxide.16–19 As we could characterize such a U(IV) species with ligands similar to aqua ligands,47 we expect that this result is important for actinoid speciation in aqueous solutions as well.
[Ti(NH3)8]Cl3·6NH3 so far has been the compound with the highest observed ammonia content in the TiX3/NH3 system (X = Cl and Br). It contains a square-antiprismatic octammine titanium(III) cation, which, to the best of our knowledge, represents the first example of this coordination number with monodentate homoleptic ligands on Ti. Also, the compound seems to be the first example of an octaammine complex of a transition metal. Interestingly, the titanium(III) compound appears colorless to the eye. As it is unstable towards the loss of ammonia above −40 °C, further analytic methods were hampered. Quantum chemical calculations show that the absorption in [Ti(NH3)8]3+ is clearly red-shifted in comparison to the absorption of the well-known [Ti(H2O)6]3+, resulting in a pale blue complementary color that might be difficult to observe with the naked eye.
Titanium(III)-chloride seems to be a fluoride ion acceptor usable for the liquid ammonia system. It is able to subtract three fluoride ions from UF4 and green crystals of [UF(NH3)8]Cl3·3.5NH3 are formed as a product. The [UF(NH3)8]3+ cation is best described as a distorted, trifold-capped trigonal prism. Despite the coordination number of nine, the U–F-distance is quite short with 2.117(1) Å, thus showing the high Lewis-acidity of U(IV). The compound seems to be the first example of a mononuclear uranium(IV) complex of coordination number nine with inorganic monodentate ligands. Due to the similarity of the aqueous and the ammonia solvent systems, we believe that the existence of such a species in liquid ammonia is an important addition and aid for the knowledge and detection of actinoids in aqueous solutions. Possibly, the respective aquo complex [UF(H2O)8]3+ can also be detected in dilute aqueous solutions from which UF4 or its hydrates do not precipitate yet.
Notes and references
- I. Castro-Rodriguez, H. Nakai, L. N. Zakharov, A. L. Rheingold and K. Meyer, Science, 2004, 305, 1757 CrossRef CAS PubMed.
- A. R. Fox, S. C. Bart, K. Meyer and C. C. Cummins, Nature, 2008, 455, 341 CrossRef CAS PubMed.
- S. M. Mansell, F. Bonnet, M. Visseaux and P. L. Arnold, Dalton Trans., 2013, 42, 9033 RSC.
- K. E. Knope and L. Soderholm, Chem. Rev., 2013, 113, 944 CrossRef CAS PubMed.
- V. Doetsch, J. Jander, U. Engelhardt, C. Lafrenz, J. Fischer, H. Nagel, W. Renz, G. Türk, T. von Volkmann and G. Weber, Chemistry in Nonaqueous Ionizing Solvents – Volume I Chemistry in Anhydrous Liquid Ammonia – Part 1 Anorganische Und Allgemeine Chemie in Flüssigem Ammoniak, Friedr. Vieweg & Sohn, Braunschweig, 1966 Search PubMed.
- S. C. Bart and K. Meyer, in Structure and Bonding 127: Organometallic and Coordination Chemistry of the Actinides, Springer-Verlag, Berlin, Heidelberg, 2008, pp. 119 Search PubMed.
- J. L. Sessler, P. J. Melfi and G. D. Pantos, Coord. Chem. Rev., 2006, 250, 816 CrossRef CAS PubMed.
- S. A. Cotton, Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 2011, 107, 264 RSC.
- M. B. Jones and A. J. Gaunt, Chem. Rev., 2013, 113, 1137 CrossRef CAS PubMed.
- S. R. Daly and G. S. Girolami, Inorg. Chem., 2010, 49, 5157 CrossRef CAS PubMed.
- S. R. Daly and G. S. Girolami, Chem. Commun., 2010, 46, 407 RSC.
- S. R. Daly, M. Ephritikhine and G. S. Girolami, Polyhedron, 2012, 33, 41 CrossRef CAS PubMed.
- H. Brumm, E. Peters and M. Jansen, Angew. Chem., Int. Ed., 2001, 40, 2069 CrossRef CAS.
- D. M. Young, G. L. Schimek and J. W. Kolis, Chem. Informationsdienst, 1997, 28, 12 Search PubMed.
- C. C. Quitmann and K. Müller-Buschbaum, Z. Anorg. Allg. Chem., 2005, 631, 564 CrossRef CAS PubMed.
- J. Maynadie, J.-C. Berthet, P. Thuery and M. Ephritikhine, Cambridge Crystallographic Data Centre, Private Communication, 2013, ZIXLEZ.
- N. Koshino, Y. Kachi, T. R. Varga, A. C. Benyei, M. Shiro, K. Takao and Y. Ikeda, Inorg. Chim. Acta, 2009, 362, 3433 CrossRef CAS PubMed.
- M.-J. Crawford, A. Ellern, K. Karaghiosoff and P. Mayer, Inorg. Chem., 2009, 48, 10877 CrossRef CAS PubMed.
- J.-C. Berthet, P. Thuery and M. Ephritikhine, Inorg. Chem., 2005, 1142 CrossRef CAS.
- F. Kraus and S. A. Baer, Chem. – Eur. J., 2009, 15, 8269–8274 CrossRef CAS PubMed.
- F. Kraus, BioInorg. React. Mech., 2012, 8, 29 CAS.
- F. Kraus, S. A. Baer, M. R. Buchner and A. J. Karttunen, Chem. – Eur. J., 2012, 18, 2131 CrossRef CAS PubMed.
- R. C. Fay, Coord. Chem. Rev., 1981, 37, 9 CrossRef CAS.
- R. C. Fay, Coord. Chem. Rev., 1982, 45, 9 CrossRef CAS.
- M. Hartmann, T. Clark and R. van Eldik, J. Phys. Chem. A, 1999, 103, 9899 CrossRef CAS.
- T. Sichla, R. Niewa, U. Zachwieja, R. Eámann and H. Jacobs, Z. Anorg. Allg. Chem., 1996, 622, 2074 CrossRef CAS PubMed.
- R. B. English and D. J. Eve, Inorg. Chim. Acta, 1993, 203, 219 CrossRef CAS.
- X.-Q. Zhang, B. Xu, Y.-H. Li and W. Li, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, m437 CAS.
- C. G. Richter and W. Jeitschko, J. Solid State Chem., 1997, 134, 26 CrossRef CAS.
- U. D. Scholz and W. Jeitschko, Z. Anorg. Allg. Chem., 1986, 540, 234 CrossRef PubMed.
- M.-H. Xie, X.-L. Yang, Y. He, J. Zhang, B. Chen and C.-D. Wu, Chem. – Eur. J., 2013, 19, 14316 CrossRef CAS PubMed.
- P. Woidy, A. J. Karttunen, T. G. Müller and F. Kraus, Z. Naturforsch., 2014, 69b, 1141 Search PubMed.
- R. E. Johnsen, P. B. Jensen, P. Norby and T. Vegge, J. Phys. Chem. C, 2014, 118, 24349 CAS.
- N. Korber and J. Daniels, Z. Anorg. Allg. Chem., 1999, 625, 189 CrossRef CAS.
- K. Müller-Buschbaum, Z. Anorg. Allg. Chem., 2007, 633, 1403 CrossRef PubMed.
- H. L. Schläfer and W. Schroeder, Z. Anorg. Allg. Chem., 1966, 347, 45 CrossRef PubMed.
- W. C. Schumb and R. F. Sundström, J. Am. Chem. Soc., 1933, 55, 596 CrossRef CAS.
- H. Goerges and A. Stähler, Ber. Dtsch. Chem. Ges., 1909, 42, 3200 CrossRef PubMed.
- J. K. Burdett, R. Hoffmann and R. C. Fay, Inorg. Chem., 1978, 17, 2553 CrossRef CAS.
- C. Kepert, Prog. Inorg. Chem., 1978, 24, 179 CrossRef PubMed.
- C. Plitzko, M. Strecker and G. Meyer, Z. Anorg. Allg. Chem., 1997, 623, 79 CrossRef CAS PubMed.
- F. Kraus, S. A. Baer and M. B. Fichtl, Eur. J. Inorg. Chem., 2009, 441 CrossRef CAS PubMed.
- H. L. Schläfer and W. Schroeder, Z. Anorg. Allg. Chem., 1966, 347, 59 CrossRef PubMed.
- S. Maurelli, S. Livraghi, M. Chiesa, E. Giamello, S. Van Doorslaer, C. Di Valentin and G. Pacchioni, Inorg. Chem., 2011, 50, 2385 CrossRef CAS PubMed.
- A. F. Holleman and N. Wiberg, Lehrbuch Der Anorganischen Chemie, Walter De Gruyter, Berlin, 2007 Search PubMed.
- P. Woidy, A. J. Karttunen and F. Kraus, Z. Anorg. Allg. Chem., 2012, 638, 2044 CrossRef CAS PubMed.
- C. Apostolidis, B. Schimmelpfennig, N. Magnani, P. Lindqvist-Reis, O. Walter, R. Sykora, A. Morgenstern, E. Colineau, R. Caciuffo and R. Klenze, et al. , Angew. Chem., Int. Ed., 2010, 49, 6343 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, crystallographic details, hydrogen bonding, and computational information. See DOI: 10.1039/c5cc04411a |
| This journal is © The Royal Society of Chemistry 2015 |