Open Access Article
Open Access ArticleSupramolecular hydrophobic guest transport system based on pillar[5]arene†
Zhenhui
Qi
,
Katharina
Achazi
,
Rainer
Haag
,
Shengyi
Dong
* and
Christoph A.
Schalley
*
Institut für Chemie und Biochemie, Freie Universität Berlin, Takustrasse 3, 14195 Berlin, Germany. E-mail: s.dong@schalley-lab.de; c.schalley@fu-berlin.de; Fax: +49 30-838-55817; Tel: +49 30-838-52639
First published on 22nd May 2015
Abstract
A pillar[5]arene-based bioactive guest loading system has been developed, which can increase the solubility of the drug norharmane in aqueous medium, and also enable its pH-stimulated release. Furthermore, this supramolecular transport system reduces the toxicity of loaded guest.
The development of efficient and responsive guest-loading complexes is not only of fundamental scientific interest, but also exerts significant value for pharmaceutical applications.1 Supramolecular structures involving macrocycles have been an intense area of research, because they serve as models for understanding natural supramolecular self-assemblies and molecular recognition motifs, and more importantly, they provide precursors for designing novel nanomaterials for biomedical applications.2 Currently, there is an urgent need for methods that improve the solubility of poorly soluble bioactive candidates, because an estimated 40–70% of new chemical entities belong to Biopharmaceutics Classification System (BCS) Class II, which comprises compounds with low solubility, but high intestinal permeability.3 Supramolecular entities, such as host–guest complexes,4 supramolecular polymers,5 vesicles and micelles,6 have gained increased attention in applications as guest transport systems. These entities not only trap high-energy forms of bioactive ingredients owing to their high binding constant (Ka > 104 M−1), but also expose dynamical, stimuli-responsive, and controllable guest-recognition behaviour, which enables the release of molecules under external stimuli.
Pillar[n]arenes (P[n]s) are a new class of macrocyclic hosts beside crown ethers,7 cyclodextrins,8 calixarenes,9 and cucurbiturils.10 The repeating units of P[n]s are connected by methylene bridges in the two para-positions, forming rigid pillar-like architectures.11 The unique structures and easy functionalization of pillararenes have given them outstanding abilities to selectively bind different kinds of guests and provided a useful platform for the construction of many interesting supramolecular systems, including cyclic dimers,12 chemosensors,13 supramolecular polymers,14 and transmembrane channels.15 However, exploring P[n]s' applications in biomedical fields, particularly as a drug loading system, is still in its infancy.16 The main reason can be ascribed to the limited information available on the biocompability of P[n]s. Also, little effort has been devoted to gaining insight on their host–guest complexation behaviour with bioactive molecules.17 Just recently, for example, Huang's group reported a pillararene-based ternary drug-delivery system with photo-controlled anticancer drug release.17a Wang et al. described P[6]-derived vesicles that can entrap model drugs and realize controlled release.17b,c In these P[n]s-based loading systems, the main design strategy was to introduce suitable guest molecules to construct supramolecular amphiphiles along with P[n]s via host–guest chemistry, which could then further assemble into micelles/vesicles. The model drugs were loaded during the preparation of these assemblies and then exposed to dialysis. Apparently, this type of formulation strategy inevitably required several synthetic steps for host/guest molecules as well as additional purification processes. Moreover, the preparation of large-scale amounts was probably going to be even more costly. To realize loading and release on demand, a simpler, more economic loading system was needed in which the P[n]s could directly interact with bioactive molecules.
Herein, we report a supramolecular approach to induce drug transport based on a water-soluble pillar[5]arene. The molecular recognition event facilitated a more soluble hydrophobic drug molecule in aqueous solution. The reversible complexation and decomplexation with drug molecules could be easily monitored and controlled by pH variation.
As depicted in Scheme 1, two water-soluble pillar[5]arene hosts 1 and 2 were chosen for this study. The synthesis of 1 and 2 was prepared by a “building-block approach” from two well-known key intermediates that were available from inexpensive starting materials.18 Norharmane (NHM) was selected as a hydrophobic drug model to investigate the ability of the loading capacity and potential host–guest properties of 1 and 2. NHM, known also as β-carboline, is a bioactive alkaloid, which widely exists in animals and plants,19 and has been reported to exhibit significant antioxidant abilities and neuroprotective effects against neurotoxins20 and potent antitumor, antiviral, antimicrobial, and antiparasitic activities in photodynamic therapy.21 In our test approach, we observed a large excess of insoluble solid NHM (2 mg) floating in the tested vial (1 mL H2O). However, the excess insoluble NHM could be rapidly dissolved when the certain amount of 1 (20 mg) was added to the system under gentle stirring (1 min, 500 rpm), which suggests host 1 was able to facilitate the solvation process of hydrophobic bioactive molecule. Under identical conditions, however, compound 2 did not lead to any apparent solubilisation, which indicated that the terminal functional group played a vital role for the selective recognition.
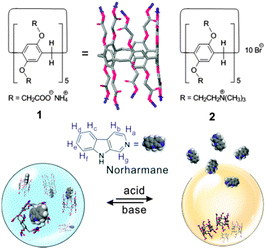 | ||
| Scheme 1 Chemical structures of water-soluble pillar[5]arenes 1 and 2, and bioactive model molecule norharmane (NHM). | ||
The 1H NMR was recorded to follow the solubilisation process. As shown in Fig. 1, we found that the proton signals of NHM clearly showed upfield shifts upon addition of 1. For example, the pyridine ring proton (Ha) shifted from 8.82 ppm to 8.16 ppm, and Hd on the indole moiety moved from 8.25 ppm to 7.70 ppm, respectively. All these signal changes were consistent with the shielding effect observed in other NHM-involved complexes.22 As expected, the addition of 2 to the suspension of NHM did not cause any significant variation in the 1H NMR spectra (Fig. S1, ESI†). The detailed phase-solubility diagram (Fig. 1, plot of [NHM] versus [1]) shows the solubility of the drug molecule was thus increased by factor of 100 (up to 4.5 mg mL−1) with the assistance of pillararene host (Fig. S3, ESI†).
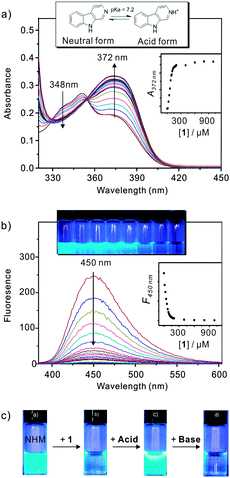
We gained more detailed recognition information between host 1 and NHM through UV-vis and fluorescence examinations, since NHM is also known for its excellent chemosensory response and has been widely used as a molecular probe for biophysics studies.23 In aqueous medium, the absorption spectrum of saturated NHM solution showed two characteristic bands with maxima at 348 nm and 372 nm that correspond to its neutral and cationic forms, respectively (Fig. 2a). With gradual addition of 1, the band belonging to the neutral form of NHM concomitantly decreased, and the band belonging to the acid form increased, which is due to the shifted ground state of NHM from prototropic equilibrium towards its cationic form. In a fluorescent study, we observed that the blue emission band at 450 nm, ascribed to the cationic form of NHM, was rapidly quenched after addition of 1 (Fig. 2b). The Stern–Volmer quenching constant (Ksv), an indicator of the degree of sensitivity of quencher, was found to be as high as 1.36 × 105 M−1 in our case (Fig. S4, ESI†), which is even compatible to some reported efficient quenchers with high Ksv.24 In fact, the fluorescence quenching process usually can be caused by a collision process (dynamic quenching mechanism) or by formation of a complex between quencher and fluorophore (static quenching mechanism).
Here the obtained bimolecular quenching constant (kq) has a value of ∼1012 M−1 s−1 (Fig. S4, ESI†), which is two orders larger than the maximum value of diffusion-limited rate constant (∼1010 M−1 s−1) and indicates the observed quenching mechanism is due to complex formation. Moreover, previous studies focusing on natural β-carboline alkaloids found that the chemical groups with lower oxidation potential facilitated the fluorescence quenching since the protons transferred from the singlet-excited β-carboline to the receptor.25 Considering the relatively low oxidation potential of carboxylates, the rapid quenching process induced by 1 could be attributed to the electrostatic interaction between the cationic fluorophore (singlet-excited NHM) and the negative COO− group on 1. In other words, the NHM favourably binds to the rim region where there is a high concentration of carboxylate anion (as illustrated in Scheme 1), instead of being embedded in the cavity of 1, which is somewhat unique to many other host−guest complexes based on pillararenes.26 However, it was still difficult to determine the cavity's hydrophobic effect upon the complexation. The Job plot showed the complex adapted the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NHM·1 stoichiometry (Fig. S5, ESI†), which implied the binding of the first NHM molecule resulted in a structural alternation that was unfavourable for binding the second guest. Using the Benesi-Hildebrand equation, we determined that the binding constants Ka for NHM·1 was 3.4 × 104 M−1 (Fig. S6, ESI†).
1 NHM·1 stoichiometry (Fig. S5, ESI†), which implied the binding of the first NHM molecule resulted in a structural alternation that was unfavourable for binding the second guest. Using the Benesi-Hildebrand equation, we determined that the binding constants Ka for NHM·1 was 3.4 × 104 M−1 (Fig. S6, ESI†).
Here the pH-triggered release was achieved because of the pH-sensitivity of carboxylate group. Adding a little excess of hydrochloric acid to a mixed solution of NHM and 1 immediately resulted in a fast and obvious phase transition from a clear solution to a turbid colloid (Fig. 2c), which was ascribed to the precipitated carboxylic-acid form of pillar[5]arene 1.16c Since the fluorescent intensity of NHM under acidic conditions (pH = 1.5) is known to be approximately three times higher than in neutral medium (pH = 7.0),27 we can estimate that over 90% of the complexed drug molecules were released upon the reduction of the pH value (Fig. S7, ESI†). The further addition of ammonia changed the system to neutral medium, and the fluorescence of NHM was regained correspondingly.
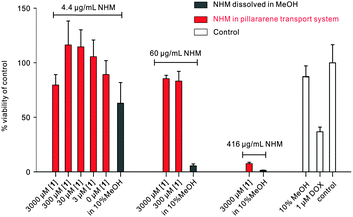
The cytotoxicity of 1 was exemplarily evaluated in different cell lines. Up to a concentration of 300 μM mL−1, no cytotoxicity could be observed in human kidney cells (HEK 293), human liver cells (HepG2) and human monocyte cells (THP-1) in a MTS assay (Fig. S8, ESI†). Furthermore, we tested in vitro cytotoxic activity of the NHM·1 system in A549 cells by MTT (3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide) for further applications. We found that the toxicity for NHM obviously decreased after supramolecular complexation (Fig. 3). For example, cells treated with concentrations of 60 μg mL−1 NHM (367 μM) showed only a minor reduction in cell viability (∼20%). In contrast, the relative cell viability of the sample treated with NHM dissolved in methanol sharply reduced (∼95%). Previous studies showed β-carboline alkaloids are characteristic for DNA binding.20b Particularly at high dose condition, the intercalation between β-carboline species into DNA may lead to conformational changes and disrupt the normal mechanism of DNA replication, transcription, and repair.21b Therefore, the observed toxicity reduction could be ascribed to the lowered chance of NHM bound to the DNA in nucleus and mitochondrion of cells. From the above results, it could be demonstrated that this supramolecular induced drug transport system diminished the toxicity of bioactive molecules even at high concentrations of NHM.
In summary, we have presented a versatile, simplified hydrophobic guest transport system based on the pillararene. The introduction of supramolecular interactions between P[5] derivative and NHM exhibited improvement of the water solubility of the bioactive molecule. Compared with individual NHM, the supramolecular transport system containing both NHM and 1 showed a significant reduction of the drug's cell toxicity, when at higher concentration. Considering that the increased numbers of P[n]s homologues (n = 6–14) contain larger cavities (in nanosize) and more functional groups,28 we believe the pillararenes-based guest transport system has great potential as drug delivery system.
This work was supported by Deutsche Forschungsgemeinschaft (SFB 765) and Focus Area Nanoscale at Freie Universität Berlin. The authors thank Christina Kühl for the assistance of cell viability experiment. Dr Shengyi Dong acknowledges financial support from the Alexander von Humboldt Foundation. The authors acknowledge Dr P. Winchester for proof reading and Dr David Mosser providing HeLa cells.
Notes and references
- (a) Z. Laughrey and B. C. Gibb, Chem. Soc. Rev., 2011, 40, 363 RSC; (b) K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394 RSC.
- (a) Z. Niu and H. W. Gibson, Chem. Rev., 2009, 109, 6024 CrossRef CAS PubMed; (b) F. Lei, M. A. Olson, D. Benitez, E. Tkatchouk, W. A. Goddard and J. F. Stoddart, Chem. Soc. Rev., 2010, 39, 17 RSC; (c) J. Wu, A. Zawistowski, M. Ehrmann, T. Yi and C. Schmuck, J. Am. Chem. Soc., 2011, 133, 9720 CrossRef CAS PubMed; (d) F. Huang and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 5879 RSC; (e) S.-L. Li, T. Xiao, C. Lin and L. Wang, Chem. Soc. Rev., 2012, 41, 5950 RSC; (f) Y. Ding, P. Wang, Y.-K. Tian and F. Wang, Chem. Commun., 2013, 49, 5951 RSC; (g) S. Dong, B. Zheng, F. Wang and F. Huang, Acc. Chem. Res., 2014, 47, 1982 CrossRef CAS PubMed; (h) G. Ghale and W. M. Nau, Acc. Chem. Res., 2014, 47, 2150 CrossRef CAS PubMed; (i) Z. Qi and C. A. Schalley, Acc. Chem. Res., 2014, 47, 2222 CrossRef CAS PubMed; (j) G. Stephenson, R. M. Parker, Y. Lan, Z. Yu, O. A. Scherman and C. Abell, Chem. Commun., 2014, 50, 7048 RSC; (k) J. A. Foster and J. W. Steed, Angew. Chem., Int. Ed., 2010, 49, 6718 CrossRef CAS PubMed.
- (a) C. A. Lipinski, J. Pharmacol. Toxicol. Methods, 2000, 44, 235 CrossRef CAS; (b) D. J. Hauss, Adv. Drug Delivery Rev., 2007, 59, 667 CrossRef CAS PubMed; (c) C. J. H. Porter, N. L. Trevaskis and W. N. Charman, Nat. Rev. Drug Discovery, 2007, 6, 231 CrossRef CAS PubMed.
- (a) J. Li and X. J. Loh, Adv. Drug Delivery Rev., 2008, 60, 1000 CrossRef CAS PubMed; (b) Y. Chen and Y. Liu, Chem. Soc. Rev., 2010, 39, 495 RSC; (c) G. Chen and M. Jiang, Chem. Soc. Rev., 2011, 40, 2254 RSC.
- (a) R. Haag, Angew. Chem., Int. Ed., 2004, 43, 278 CrossRef CAS PubMed; (b) T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed; (c) S. Biswas, K. Kinbara, T. Niwa, H. Taguchi, N. Ishii, S. Watanabe, K. Miyata, K. Kataoka and T. Aida, Nat. Chem., 2013, 5, 613 CrossRef CAS PubMed.
- (a) S. Nowag and R. Haag, Angew. Chem., Int. Ed., 2014, 53, 49 CrossRef CAS PubMed; (b) Y. Wang, S. Song, J. Liu, D. Liu and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 536 CAS.
- (a) S. J. Loeb and J. A. Wisner, Angew. Chem., Int. Ed., 1998, 37, 2838 CrossRef CAS; (b) H. W. Gibson, N. Yamaguchi, L. Hamilton and J. W. Jones, J. Am. Chem. Soc., 2002, 124, 4652 Search PubMed; (c) F. Huang, F. R. Fronczek and H. W. Gibson, J. Am. Chem. Soc., 2003, 125, 9272 CrossRef CAS PubMed; (d) H. W. Gibson, N. Yamaguchi and J. W. Jones, J. Am. Chem. Soc., 2003, 125, 3522 CrossRef CAS PubMed; (e) W. Jiang and C. A. Schalley, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10425 CrossRef CAS PubMed; (f) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed; (g) L. Chen, Y.-K. Tian, Y. Ding, Y.-J. Tian and F. Wang, Macromolecules, 2012, 45, 8412 CrossRef CAS; (h) Z. Qi, P. Malo de Molina, W. Jiang, Q. Wang, K. Nowosinski, A. Schulz, M. Gradzielski and C. A. Schalley, Chem. Sci., 2012, 3, 2073 RSC; (i) Z. Qi, C. Wu, P. Malo de Molina, H. Sun, A. Schulz, C. Griesinger, M. Gradzielski, R. Haag, M. B. Ansorge-Schumacher and C. A. Schalley, Chem. – Eur. J., 2013, 19, 10150 CrossRef CAS PubMed; (j) Z. Qi, N. L. Traulsen, P. Malo de Molina, C. Schlaich, M. Gradzielski and C. A. Schalley, Org. Biomol. Chem., 2014, 12, 503 RSC; (k) Y.-K. Tian, Z.-S. Yang, X.-Q. Lv, R.-S. Yao and F. Wang, Chem. Commun., 2014, 50, 9477 RSC.
- A. Harada, Acc. Chem. Res., 2001, 34, 456 CrossRef CAS PubMed.
- (a) J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780 CrossRef CAS PubMed; (b) D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907 RSC.
- (a) K. Kim, Chem. Soc. Rev., 2002, 31, 96 RSC; (b) B. Vinciguerra, L. Cao, J. R. Cannon, P. Y. Zavalij, C. Fenselau and L. Isaacs, J. Am. Chem. Soc., 2012, 134, 13133 CrossRef CAS PubMed; (c) D. Ma, G. Hettiarachchi, D. Nguyen, B. Zhang, J. B. Wittenberg, P. Y. Zavalij, V. Briken and L. Isaacs, Nat. Chem., 2012, 4, 503 CrossRef CAS PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed; (b) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721 CrossRef CAS PubMed; (c) Z. Zhang, B. Xia, C. Han, Y. Yu and F. Huang, Org. Lett., 2010, 12, 3285 CrossRef CAS PubMed; (d) C. Li, Q. Xu, J. Li, F. Yao and X. Jia, Org. Biomol. Chem., 2010, 8, 1568 RSC; (e) M. Holler, N. Allenbach, J. Sonet and J.-F. Nierengarten, Chem. Commun., 2012, 48, 2576 RSC; (f) X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia and C. Li, Chem. Commun., 2012, 48, 2967–2969 RSC; (g) M. P. Sonawane, J. Jacobs, J. Thomas, L. V. Meervelt and W. Dehaen, Chem. Commun., 2013, 49, 6310 RSC; (h) T. Adiri, D. Marcianob and Y. Cohen, Chem. Commun., 2013, 49, 7082 RSC; (i) H. Zhang, X. Ma, J. Guo, K. T. Nguyen, Q. Zhang, X.-J. Wang, H. Yan, L. Zhu and Y. Zhao, RSC Adv., 2013, 3, 368 RSC; (j) C. Li, J. Ma, L. Zhao, Y. Zhang, Y. Yu, X. Shu, J. Li and X. Jia, Chem. Commun., 2013, 49, 1924 RSC; (k) L. Wu, Y. Fang, Y. Jia, Y. Yang, J. Liao, N. Liu, X. Yang, W. Feng, J. Ming and L. Yuan, Dalton Trans., 2014, 43, 3835 RSC; (l) D.-D. Zheng, D.-Y. Fu, Y. Wu, Y.-L. Sun, L.-L. Tan, T. Zhou, S.-Q. Ma, X. Zha and Y.-W. Yang, Chem. Commun., 2014, 50, 3201 RSC; (m) Y.-W. Yang, Y.-L. Sun and N. Song, Acc. Chem. Res., 2014, 47, 1950 CrossRef CAS PubMed; (n) H. Chen, J. Fan, X. Hu, J. Ma, S. Wang, J. Li, Y. Yu, X. Jia and C. Li, Chem. Sci., 2015, 6, 197 RSC.
- (a) Z. Zhang, G. Yu, C. Han, J. Liu, X. Ding, Y. Yu and F. Huang, Org. Lett., 2011, 13, 4818 CrossRef CAS PubMed; (b) X. Wang, K. Han, J. Li, X. Jia and C. Li, Polym. Chem., 2013, 4, 3998 RSC; (c) K. Wang, C.-Y. Wang, Y. Zhang, S. X.-A. Zhang, B. Yang and Y.-W. Yang, Chem. Commun., 2014, 50, 9458 RSC.
- G. Yu, Z. Zhang, C. Han, M. Xue, Q. Zhou and F. Huang, Chem. Commun., 2012, 48, 2958 RSC.
- (a) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397 CrossRef CAS PubMed; (b) B. Xia, B. Zheng, C. Han, S. Dong, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 2019 RSC; (c) X. Hu, X. Wu, S. Wang, D. Chen, W. Xia, C. Lin, Y. Pan and L. Wang, Polym. Chem., 2013, 4, 4292 RSC; (d) X. Wang, H. Deng, J. Li, K. Zheng, X. Jia and C. Li, Macromol. Rapid Commun., 2013, 34, 1856 CrossRef CAS PubMed; (e) K. Wang, C.-Y. Wang, Y. Wang, H. Li, C.-Y. Bao, J.-Y. Liu, S. X.-A. Zhang and Y.-W. Yang, Chem. Commun., 2013, 49, 10528 RSC; (f) C. Li, K. Han, J. Li, Y. Zhang, W. Chen, Y. Yu and X. Jia, Chem. – Eur. J., 2013, 19, 11892 CrossRef CAS PubMed; (g) Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577 CrossRef CAS PubMed; (h) N. Song, D.-X. Chen, Y.-C. Qiu, X.-Y. Yang, B. Xu, W. Tian and Y.-W. Yang, Chem. Commun., 2014, 50, 8231 RSC; (i) C. Li, Chem. Commun., 2014, 50, 12420 RSC; (j) N. Song, D.-X. Chen, M.-C. Xia, X.-L. Qiu, K. Ma, B. Xu, W. Tian and Y.-W. Yang, Chem. Commun., 2015, 51, 5526 RSC.
- W. Si, L. Chen, X.-B. Hu, G. Tang, Z. Chen, J.-L. Hou and Z.-T. Li, Angew. Chem., Int. Ed., 2011, 50, 12564 CrossRef CAS PubMed.
- (a) Y.-L. Sun, Y.-W. Yang, D.-X. Chen, G. Wang, Y. Zhou, C.-Y. Wang and J. F. Stoddart, Small, 2013, 9, 3224–3229 CAS; (b) H. Zhang, X. Ma, K. T. Nguyen and Y. Zhao, ACS Nano, 2013, 7, 7853 CrossRef CAS PubMed; (c) D.-D. Zheng, D.-Y. Fu, Y. Wu, Y.-L. Sun, L.-L. Tan, T. Zhou, S.-Q. Ma, X. Zha and Y.-W. Yang, Chem. Commun., 2014, 50, 3201 RSC; (d) P. Wang, Z. Li and X. Ji, Chem. Commun., 2014, 50, 13114 RSC.
- (a) G. Yu, W. Yu, Z. Mao, C. Gao and F. Huang, Small, 2015, 11, 919 CrossRef CAS PubMed; (b) Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS PubMed; (c) Y. Cao, X.-Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y.-Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762 CrossRef CAS PubMed.
- (a) T. Ogoshi, M. Hashizume, T.-A. Yamagishi and Y. Nakamoto, Chem. Commun., 2010, 46, 3708 RSC; (b) Y. Ma, X. Ji, F. Xiang, X. Chi, C. Han, J. He, Z. Abliz, W. Chen and F. Huang, Chem. Commun., 2011, 47, 12340 RSC.
- K. Touiki, P. Rat, R. Molimard, A. Chait and R. de Beaurepaire, Psychopharmacology, 2005, 182, 562 CrossRef CAS PubMed.
- (a) H. Haghdoost-Yazdi, S.-S. Hosseini, A. Faraji, D. Nahid and H. Jahanihashemi, Behav. Brain Res., 2010, 215, 136 CrossRef CAS PubMed; (b) M. M. Gonzalez, M. Pellon-Maison, M. A. Ales-Gandolfo, M. R. Gonzalez-Baro, R. Erra-Balsells and F. M. Cabrerizo, Org. Biomol. Chem., 2010, 8, 2543 RSC.
- (a) R. Cao, W. Peng, Z. Wang and A. Xu, Curr. Med. Chem., 2007, 14, 479 CrossRef CAS; (b) C. de Meester, Mutat. Res., 1995, 339, 139 CrossRef CAS.
- A. Mallick, T. Katayama, Y. Ishibasi, M. Yasuda and H. Miyasaka, Analyst, 2011, 136, 275 RSC.
- A. Chakrabarty, A. Mallick, B. Haldar, P. Das and N. Chattopadhyay, Biomacromolecules, 2007, 8, 920 CrossRef CAS PubMed.
- A. Mallick, M. C. Mandal, B. Haldar, A. Chakrabarty, P. Das and N. Chattopadhyay, J. Am. Chem. Soc., 2006, 128, 3126 CrossRef CAS PubMed.
- Z. Miskolczy, M. Megyesi, L. Biczok and H. Gorner, Photochem. Photobiol. Sci., 2011, 10, 592 CAS.
- (a) P. Wang, Y. Yao and M. Xue, Chem. Commun., 2014, 50, 5064 RSC; (b) X. Chi, M. Xue, Y. Ma, X. Yan and F. Huang, Chem. Commun., 2013, 49, 8175 RSC; (c) W. Chen, Y. Zhang, J. Li, X. Lou, Y. Yu, X. Jia and C. Li, Chem. Commun., 2013, 49, 7956 RSC; (d) J. Fan, H. Deng, J. Li, X. Jia and C. Li, Chem. Commun., 2013, 49, 6343 RSC.
- F. Tomas Vert, I. Zabala Sanchez and A. Olba Torrent, J. Photochem., 1983, 23, 355 CrossRef.
- T. Ogoshi, N. Ueshima, F. Sakakibara, T.-A. Yamagishi and T. Haino, Org. Lett., 2014, 16, 2896 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterization, association constant determination, and other materials. See DOI: 10.1039/c5cc03955j |
| This journal is © The Royal Society of Chemistry 2015 |