Open Access Article
Open Access ArticleA new Keggin-like niobium-phosphate cluster that reacts reversibly with hydrogen peroxide†
Jung-Ho
Son
*a and
William H.
Casey
ab
aDepartment of Chemistry, University of California, Davis, One Shields Ave. Davis, CA 95616, USA. E-mail: junghoson@gmail.com
bDepartment of Chemistry, Department of Earth and Planetary Sciences, University of California, Davis, One Shields Ave. Davis, CA 95616, USA
First published on 25th June 2015
Abstract
Polyoxoniobate clusters that are stable in acidic solutions are rare and particularly useful in industrial processes. Here we report a new pentaphosphate niobate polyoxometalate cluster (TMA)9H3Nb9P5O41·28H2O (Nb9P5) that is stable over a wide pH range and that can be converted reversibly into the peroxo form.
The layered niobium-phosphate hydrate (NbOPO4·xH2O) is a useful catalyst for biomass conversion, such as the conversion of sugars to 5-hydroxymethylfurfural (HMF) and other dehydration reactions.1 Its structure consists of corner-shared NbO6 and PO4 units2 and the coexistence of both basic and acidic sites in NbOPO4·xH2O is responsible for its catalytic activity.3 Although niobium phosphate is widely used as a catalyst, its solution chemistry is rarely studied. Among the previous Keggin-type polyoxoniobates,4 Nyman et al. reported phosphate-decorated lacunary Keggin-type polyoxoniobate as an insoluble sodium salt, Na15[(PO2)3PNb9O34]·22H2O (Nb9P4), which was characterized by crystallography and MAS NMR.5 Recently we synthesized sets of peroxoniobophosphate clusters as water-soluble precursors for electron-beam-patterned niobium-phosphate films.6 Among them, the [HNb4P2O14(O2)4]5− cluster (peroxo-Nb4P2) is stable over a wide pH range, spanning into acidic conditions, which is remarkable in itself because polyoxoniobates are generally only stable and soluble in neutral to basic solutions. We here report the synthesis, structure and characterization of a Keggin-like mixed niobium-phosphate cluster as a highly soluble tetramethylammonium (TMA) salt, (TMA)9H3Nb9P5O41·28H2O (Nb9P5). The new cluster Nb9P5 features a central phosphate and four additional phosphates in the outer layers.
The Nb9P5 cluster was synthesized by hydrothermal reaction of hydrous niobium oxide, TMAOH and phosphoric acid. The solution pH is a key factor for the formation of Nb9P5. In the given synthesis condition (see ESI†), Nb9P5 forms exclusively and the solution pH = 9 after reaction. When a larger amount of TMAOH was used to make pH = 10 after reaction, a mixture of Nb9P5 and Keggin-type [PNb14O42]9− cluster (PNb14) formed,7 as we determined by electrospray-ionization mass spectrometry (ESI-MS). The PNb14 dominated when the pH of the reaction solution was higher than 11.
The shape of Nb9P5 is similar to the Keggin-type cluster, but it possesses no symmetry [Fig. 1]. There are some irregularly shaped polyoxoniobate clusters reported previously.8 Symmetry is lost in Nb9P5 because of the multiple substitutions of PO4 groups for the different NbO6 sites in the normal Keggin structure. The structure of Nb9P5 is significantly different from Nb9P4, which possesses pseudo-C3 symmetry.5 The previous Nb9P4 structure has three neighboring phosphate groups bound to the lacunary-Keggin PNb9 unit and there are two coordinating Na+ ions between the phosphate groups. Examples of similar mixed-addenda polyoxometalates with group 15 elements include an arsenate-containing polyoxovanadate.9 In the structure of Nb9P5, there are four metal-oxo triads bonded to the central phosphate. The basal plane of the Nb9P5 cluster in Fig. 1 (farthest from the viewer) is a regular Nb3O13 triad, which is a common building unit in Keggin ions. The other three triads are Nb2PO12 moieties. The two Nb2PO12 triads are in a similar orientation, with P3 and P4 coordinating the basal Nb3O13 triad, while the other Nb2PO12 triad that contains P2 is rotated about 120° counterclockwise relative to the others. These three Nb2PO12 triads are connected by an additional phosphate (P1) at the top of the Nb9P5 cluster (closest from viewer). In the crystal structure, nine TMA countercations are found and this number of TMA agrees with elemental analysis result. Thus three charge-balancing protons should be bound on the cluster to make the formula as [H3Nb9P5O41]9−. The positions of the protons could not be determined, by either crystallography nor by bond-valence sum calculation of the oxygen atoms.
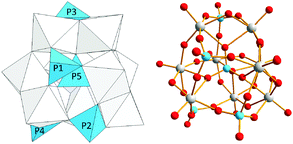 | ||
| Fig. 1 Polyhedral (left) and ball-and-stick (right) drawing of Nb9P5 cluster (Nb: gray, P: blue, O: red). | ||
The ESI-MS of the Nb9P5 cluster [Fig. S1, ESI†] exhibit a relatively complex multi-peak pattern when compared to simpler spectra of other substituted polyoxoniobate clusters that we have synthesized.6,7,10 There are multiple side peaks near the assigned peaks, which could be due to loss or addition of water from the Nb9P5 cluster during the ionization or drying steps in the ESI-MS analysis. Multiple side peaks in ESI-MS were also observed for [H2MnIVNb10O32]8− cluster.11 There are also some unassigned peaks between 800< m/z < 950, which must be due to fragmentation of Nb9P5.
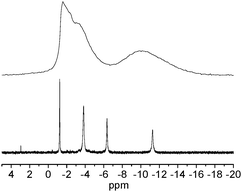
The 31P NMR spectrum of an aqueous Nb9P5 solution shows four phosphate peaks assignable to the cluster [Fig. 2]. The integrated peak areas, from left to right, are approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which agree with five different phosphate groups in the cluster stoichiometry. We hypothesized that the average P–O lengths within PO4 groups [Fig. S2, ESI†] and 31P chemical shifts are related. Assuming that the chemical shift depends on the average P–O lengths to the nucleus, we assign the peak at −1.2 ppm as the central phosphate (P5), the peak at −3.8 ppm as P1 and P4. These P1 and P4 have similar average P–O lengths, so the merged peak in the NMR spectra is understandable if the chemical shieldings were also similar. Accordingly, we assign the peak at −6.4 as P2, and the most upfield peak at −11.3 ppm as P3. The peak assignment is also in agreement with the peak widths. The P3 has the widest range of P–O lengths in the X-ray structure, and also the broadest peak in the NMR spectra, and the central P5 has the most regular P–O lengths and correspondingly the sharpest peak. The correlation suggests that asymmetry of the 31P nuclei are reflected in the spectra. The 31P MAS NMR spectra shows three overlapping peaks (−1.6, −2.4, and −3.2 ppm) in the more downfield region and a broader upfield peak at −10.1 ppm [Fig. 2]. Deconvolution and integration of the peaks gives approximate ratios of 1
1, which agree with five different phosphate groups in the cluster stoichiometry. We hypothesized that the average P–O lengths within PO4 groups [Fig. S2, ESI†] and 31P chemical shifts are related. Assuming that the chemical shift depends on the average P–O lengths to the nucleus, we assign the peak at −1.2 ppm as the central phosphate (P5), the peak at −3.8 ppm as P1 and P4. These P1 and P4 have similar average P–O lengths, so the merged peak in the NMR spectra is understandable if the chemical shieldings were also similar. Accordingly, we assign the peak at −6.4 as P2, and the most upfield peak at −11.3 ppm as P3. The peak assignment is also in agreement with the peak widths. The P3 has the widest range of P–O lengths in the X-ray structure, and also the broadest peak in the NMR spectra, and the central P5 has the most regular P–O lengths and correspondingly the sharpest peak. The correlation suggests that asymmetry of the 31P nuclei are reflected in the spectra. The 31P MAS NMR spectra shows three overlapping peaks (−1.6, −2.4, and −3.2 ppm) in the more downfield region and a broader upfield peak at −10.1 ppm [Fig. 2]. Deconvolution and integration of the peaks gives approximate ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for (−1.6 ppm and −2.4 ppm): −3.2 ppm: −10.1 ppm peaks. The peaks at −1.6 and −2.4 ppm are assigned to the central P5. Similarly to the solution 31P NMR peak assignment, the −3.2 ppm peak is assigned to P1 and P4, and the peak at −10.1 ppm is assigned to P2 and P3. In the 31P MAS NMR study of Nb9P4, two broad peaks were observed at 6.1 and 1.4 ppm, and these are assigned to outer phosphate and central phosphate groups, respectively.5 We speculate that the considerable downfield shift of the outer phosphate group in Nb9P4 compared to Nb9P5 can be due to the coordination of Na+.
2 for (−1.6 ppm and −2.4 ppm): −3.2 ppm: −10.1 ppm peaks. The peaks at −1.6 and −2.4 ppm are assigned to the central P5. Similarly to the solution 31P NMR peak assignment, the −3.2 ppm peak is assigned to P1 and P4, and the peak at −10.1 ppm is assigned to P2 and P3. In the 31P MAS NMR study of Nb9P4, two broad peaks were observed at 6.1 and 1.4 ppm, and these are assigned to outer phosphate and central phosphate groups, respectively.5 We speculate that the considerable downfield shift of the outer phosphate group in Nb9P4 compared to Nb9P5 can be due to the coordination of Na+.
The Nb9P5 was also characterized via FT-IR and Raman spectroscopy [Fig. S3, ESI†]. The FT-IR spectra show multiple P–O bands in the range of 1000–1200 cm−1, which arise from different PO4 groups. The Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band is located at 900 cm−1 and weaker M–O–M bands are observed below 800 cm−1. In the Raman spectra, multiple overlapping Nb
O band is located at 900 cm−1 and weaker M–O–M bands are observed below 800 cm−1. In the Raman spectra, multiple overlapping Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands near 900 cm−1 are observed, possibly due to the asymmetric structure of Nb9P5. We assign the weak bands in 950–1100 cm−1 to different PO4 groups.
O bands near 900 cm−1 are observed, possibly due to the asymmetric structure of Nb9P5. We assign the weak bands in 950–1100 cm−1 to different PO4 groups.
The stability of the Nb9P5 cluster in different pH conditions was examined by using ESI-MS [Fig. S4, ESI†]. The pH of the 6 mM solution of Nb9P5 was 7.3, and the cluster was apparently kinetically stable in solution for many months. The Nb9P5 cluster was stable until pH 12 when titrated with TMAOH. The cluster was also kinetically stable when titrated with HCl until pH 3. The solution at pH < 3, however, became a transparent hydrogel after a few days, which indicates a slow proton-induced charge neutralization, rearrangement and condensation.
The key point is that this Nb9P5 cluster provides another example of an aqueous niobate cluster that can be processed in acidic solutions. This stability in acidic region was also observed for the peroxoniobophosphates,6 but most of other polyoxoniobate clusters tend to precipitate as hydrous niobium oxide below pH ≤ 4–5.
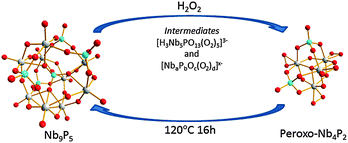
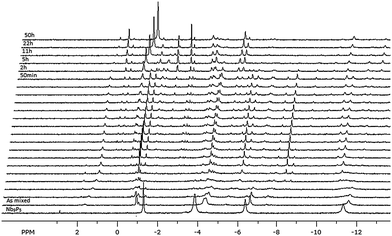
We found that the Nb9P5 cluster could be reversibly converted to the peroxo-Nb4P2 cluster [Scheme 1] when H2O2 is added. The conversion process was monitored via ESI-MS and 31P NMR after addition of the stoichiometric amount of H2O2. In the ESI-MS, we observed a set of new complex peaks with lower intensity in the m/z regions near those of the Nb9P5 [Fig. 3]. These new peaks persist for many hours after adding H2O2. This persistence suggests that peroxo-Nb9P5 or similar peroxo-Keggin-like species form. We failed to isolate these transient species for crystallization. Other new peaks centered at m/z = 288.8 grew and diminished in an hour after adding H2O2. We assign this putative new species as [H3Nb5PO13(O2)5]3−, based on m/z value [Fig. 3]. The evolution of ESI-MS peaks show that the peroxo-Nb4P2 forms exclusively with time. To further understand the reaction, 31P NMR spectra were collected after adding H2O2 [Fig. 4]. The 31P NMR peaks of Nb9P5 shift and split after addition of hydrogen peroxide, which indicates that a range of different peroxo-Nb9P5 or similar compounds form as transient intermediates. Chemical shifts of the transient species are not much different from Nb9P5, which suggests to us that the structures of the transient species can be similar to Nb9P5, but perhaps decorated differently with peroxo groups that affect the 31P chemical shifts. This observation is consistent with the ESI-MS results that the transient 31P NMR peaks show partial dissociation and rearrangement of the molecule, but not wholesale destruction. The sharp transient peak at −1 ppm is tentatively assigned to [H3Nb5PO13(O2)5]3−, which was suggested by ESI-MS.
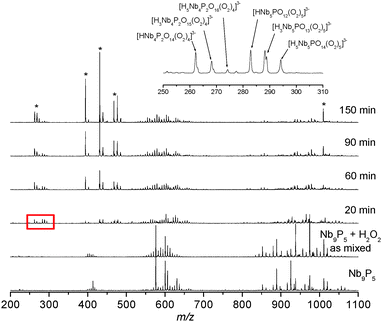 | ||
| Fig. 3 Time-dependent ESI-MS spectra of Nb9P5 solution after adding 2 equivalents of H2O2. The inset shows more detailed peak assignments. The peaks marked with asterisks correspond to peroxo-Nb4P2. | ||
We note that well resolved 31P NMR peaks are observable only when a stoichiometric amount of H2O2 is added; only a few broad peaks are observed during the reaction when more H2O2 is added, possibly due to fast speciation changes and disorder upon peroxidation. The reaction is complete after one day when slight excess H2O2 is added, but the reaction is faster when a larger excess of H2O2 is added. The growing peak of peroxo-Nb4P2 shifts upfield to −1 ppm during the reaction, which might be due to an increasing concentration of peroxo-Nb4P2. When the reaction is complete, only the peak of peroxo-Nb4P2 and a small phosphate peak near 1 ppm are observed [Fig. 4].
The reverse reaction, converting peroxo-Nb4P2 to Nb9P5, can be achieved by hydrothermal treatment of a solution of peroxo-Nb4P2 at 120 °C overnight. It is not surprising that heat destroys the peroxide moieties, but the Nb9P5 forms readily because both clusters have a similar Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio and because Nb9P5 is stable at near-neutral conditions (pH ∼7). The sensitivity of the peroxide moieties to heat suggests a strategy for laser-patterning of films.
P ratio and because Nb9P5 is stable at near-neutral conditions (pH ∼7). The sensitivity of the peroxide moieties to heat suggests a strategy for laser-patterning of films.
In summary, a new niobium-phosphate cluster is described that is stable over a wide range of solution pH. This cluster thus adds to the small library of polyoxoniobate compounds that can be processed in acidic solutions. Most interestingly, the cluster can reversibly interconvert to and from the peroxo-Nb4P2, which suggests an easy means of patterning. This new cluster may be viewed as a molecular form of the solid niobium-phosphate catalyst that is so useful in industrial applications.
This work was supported by an NSF CCI grant through the Center for Sustainable Materials Chemistry, number CHE-1102637. Additional support to JHS was via NSF-CHE-1307556 to WHC. We thank Dr Ping Yu for acquisition of 31P MAS NMR spectra. We also thank Zamirbek Akimbekov and Prof. Alexandra Navrotsky for TGA data and Jiarui Wang for XRD.
Notes and references
- (a) T. Armaroli, G. Busca, C. Carlini, M. Giuttari, A. M. R. Galletti and G. Sbrana, J. Mol. Catal. A: Chem., 2000, 151, 233–243 CrossRef CAS; (b) P. Carniti, A. Gervasini, S. Biella and A. Auroux, Catal. Today, 2006, 118, 373–378 CrossRef CAS PubMed; (c) Y. Zhang, J. J. Wang, J. Ren, X. H. Liu, X. C. Li, Y. J. Xia, G. Z. Lu and Y. Q. Wang, Catal. Sci. Technol., 2012, 2, 2485–2491 RSC; (d) R. M. West, M. H. Tucker, D. J. Braden and J. A. Dumesic, Catal. Commun., 2009, 10(13), 1743–1746 CrossRef CAS PubMed; (e) A. Florentino, P. Cartraud, P. Magnoux and M. Guisnet, Appl. Catal., A, 1992, 89, 143–153 CrossRef CAS; (f) T. Okuhara, Chem. Rev., 2002, 102, 3641–3666 CrossRef CAS PubMed; (g) V. V. Ordomsky, V. L. Sushkevich, J. C. Schouten, J. van der Schaaf and T. A. Nijhuis, J. Catal., 2013, 300, 37–46 CrossRef CAS PubMed; (h) K. Nakajima, Y. Baba, R. Noma, M. Kitano, J. N. Kondo, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2011, 133(12), 4224–4227 CrossRef CAS PubMed.
- (a) J. M. Longo and P. Kierkegaard, Acta Chem. Scand., 1966, 20, 72 CrossRef CAS PubMed; (b) K. Beneke and G. Lagaly, Inorg. Chem., 1983, 22, 1503–1507 CrossRef CAS.
- (a) S. Okazaki and A. Kurosaki, Catal. Today, 1990, 8, 113–122 CrossRef CAS; (b) S. Okazaki and N. Wada, Catal. Today, 1993, 16, 349–359 CrossRef CAS; (c) P. Carniti, A. Gervasini, S. Biella and A. Auroux, Chem. Mater., 2005, 17, 6128–6136 CrossRef CAS; (d) Y. Zhang, J. J. Wang, X. C. Li, X. H. Liu, Y. J. Xia, B. C. Hu, G. Z. Lu and Y. Q. Wang, Fuel, 2015, 139, 301–307 CrossRef CAS PubMed.
- (a) M. Nyman, F. Bonhomme, T. M. Alam, M. A. Rodriguez, B. R. Cherry, J. L. Krumhansl, T. M. Nenoff and A. M. Sattler, Science, 2002, 297, 996–998 CrossRef CAS PubMed; (b) M. Nyman, F. Bonhomme, T. M. Alam, J. B. Pairse and G. M. B. Vaughan, Angew. Chem., Int. Ed., 2004, 43, 2787–2792 CrossRef CAS PubMed; (c) F. Bonhomme, J. P. Larentzos, T. M. Alam, E. J. Maginn and M. Nyman, Inorg. Chem., 2005, 44, 1774–1785 CrossRef CAS PubMed; (d) M. Nyman, C. R. Powers, F. Bonhomme, T. M. Alam, E. J. Maginn and D. T. Hobbs, Chem. Mater., 2008, 20, 2513–2521 CrossRef CAS; (e) J.-H. Son, C. A. Ohlin, R. L. Johnson, P. Yu and W. H. Casey, Chem. – Eur. J., 2013, 19, 5191–5197 CrossRef CAS PubMed; (f) J.-H. Son, C. A. Ohlin, E. C. Larson, P. Yu and W. H. Casey, Eur. J. Inorg. Chem., 2013, 1748–1753 CrossRef CAS PubMed; (g) P. Huang, E.-L. Zhou, X.-L. Wang, C.-Y. Sun, H.-N. Wang, Y. Xing, K.-Z. Shao and Z.-M. Su, CrystEngComm, 2014, 16, 9582–9585 RSC; (h) J.-Q. Shen, Q. Wu, Y. Zhang, Z.-M. Zhang, Y.-G. Li, Y. Lu and E.-B. Wang, Chem. – Eur. J., 2014, 20, 2840–2848 CrossRef CAS PubMed; (i) J.-Q. Shen, Y. Zhang, Z.-M. Zhang, Y.-G. Li, Y.-Q. Gao and E.-B. Wang, Chem. Commun., 2014, 50, 6017–6019 RSC.
- M. Nyman, A. J. Celestian, J. B. Parise, G. P. Holland and T. M. Alam, Inorg. Chem., 2006, 45, 1043–1052 CrossRef CAS PubMed.
- J.-H. Son, D.-H. Park, D. A. Keszler and W. H. Casey, Chem. – Eur. J., 2015, 21, 6727–6731 CrossRef CAS PubMed.
- J.-H. Son and W. H. Casey, Chem. Commun., 2015, 51, 1436–1438 RSC.
- (a) D. Atencio, J. M. V. Coutinho, A. C. Doriguetto, Y. P. Mascarenhas, J. Ellena and V. C. Ferrari, Am. Mineral., 2008, 93, 81–87 CrossRef CAS; (b) R. Tsunashima, D.-L. Long, H. N. Miras, D. Gabb, C. P. Pradeep and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 113–116 CrossRef CAS PubMed.
- M. I. Khan, Q. Chen, J. Zubieta, D. Goshorn and R. C. Haushalter, Inorg. Chim. Acta, 1992, 191, 97–102 CrossRef CAS.
- (a) J.-H. Son, J. Wang, F. E. Osterloh, P. Yu and W. H. Casey, Chem. Commun., 2014, 50, 836–838 RSC; (b) J.-H. Son, J. Wang and W. H. Casey, Dalton Trans., 2014, 43, 17928–17933 RSC; (c) J.-H. Son, C. A. Ohlin and W. H. Casey, Dalton Trans., 2013, 42, 7529–7533 RSC; (d) C. A. Ohlin, E. M. Villa, J. C. Fettinger and W. H. Casey, Angew. Chem., Int. Ed., 2008, 47, 5634–5636 CrossRef CAS PubMed.
- J.-H. Son and W. H. Casey, Dalton Trans., 2013, 42, 13339–13342 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic data, ESI-MS, FT-IR, Raman and TGA data. CCDC 1062824. See DOI: 10.1039/c5cc03782d |
| This journal is © The Royal Society of Chemistry 2015 |