Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pressure-induced oligomerization of alanine at 25 °C
Chikako
Fujimoto
a,
Ayako
Shinozaki
ab,
Koichi
Mimura
b,
Tamihito
Nishida
c,
Hirotada
Gotou
d,
Kazuki
Komatsu
a and
Hiroyuki
Kagi
*a
aGeochemical Research Center, Graduate School of Science, The University of Tokyo, Hongo, Tokyo 113-0033, Japan. E-mail: kagi@eqchem.s.u-tokyo.ac.jp; Fax: +81 3 5841 4119; Tel: +81 3 5841 7625
bDepartment of Earth and Planetary Sciences, Graduate School of Environmental Studies, Nagoya University, Nagoya 464-8601, Japan
cDivision of Earth and Environmental Sciences, Graduate School of Environmental Studies, Nagoya University, Nagoya 464-8601, Japan
dInstitute for Solid State Physics, The University of Tokyo, Kashiwa, Chiba 277-8581, Japan
First published on 13th July 2015
Abstract
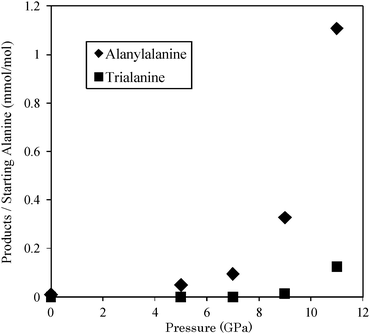
Pressure-induced oligomerization was found from high-pressure experiments at 25 °C on alanine powder soaked in its saturated aqueous solution. The oligomerization to alanylalanine occurred at 5 GPa. The maximum yields of alanylalanine and trialanine were, respectively, 1.1 × 10−3 and 1.3 × 10−4 at 11 GPa.
Intermolecular interaction changes drastically with increasing pressure, inducing chemical reactions of organic materials without a catalyst. Pressure-induced chemical reactions have been reported for several types of organic materials. For example, formation of naphthalene, biphenyl, terphenyl and so on has been reported for high-pressure experiments on benzene, a simplest aromatic hydrocarbon, up to 16 GPa at room temperature.1 Recently, crystalline one-dimensional sp3-bonded carbon nanothreads were recovered to ambient pressure from compression of benzene at 20 GPa at room temperature.2 Above that pressure, an irreversible chemical reaction occurred to form an amorphous phase.3 The overlap of π bonds and the decrease in the intermolecular distance of benzene molecules with increasing pressure induced oligomerization and amorphization,1,4 which are enhanced by increasing temperature and selective laser irradiation.4,5 Theoretical and experimental studies reported that pressure-induced oligomerization and polymerization also occurred in chain unsaturated hydrocarbons such as propene,6 butadiene,7 and acetylene.8 Furthermore, pressure-induced condensation of trimethylsilanol was observed from Raman and IR absorption spectroscopies at pressures up to 3.3 GPa at room temperature. Along with increased hydrogen bonding interaction between trimethylsilanol molecules with increasing pressure, which was suggested by notably large pressure-dependence of the OH stretching frequency (−107 cm−1 GPa−1), the condensation reaction occurred.9
In amino acids, hydrogen bonding and Coulombic interactions strongly affect their crystal structure. With increasing pressure, these interactions change drastically. Some of the amino acids undergo phase transitions.10 Pressure-dependence in the crystal structure of L-alanine up to 13.6 GPa was investigated precisely using neutron powder diffraction and single-crystal X-ray diffraction.11 Increased pressure decreases intermolecular distances, particularly those between NH3+ and COO−, and might induce chemical reactions of amino acids in addition to the continuous changes in structure and phase transformation.
Actually, high pressure can be a crucially important environment for peptide formation from amino acids. Reportedly, shock compression up to 26.3 GPa and 1170 K caused the formation of glycylglycine and triglycine from glycine.12 Static compression up to 100 MPa and 150 °C for 1–32 days results in oligomerization of glycine molecules up to 10-mer.13 Experiments under much higher pressure (1.0–5.5 GPa) and higher temperature (180–400 °C) conditions were conducted for durations from 2 h to 24 h and the formation of pentamers of glycine and alanine was reported.14 Both high temperature and high pressure were simultaneously applied to samples free from water in the previous experiments. However, it remains unclear whether heating or compression is the more important factor for oligomerization of amino acids.
To investigate pressure-induced peptide formation from amino acids at room temperature, we conducted high-pressure experiments on L-alanine by the static compression method, without heating. We analyzed the recovered samples using GC-MS and found the formation of alanylalanine and trialanine.
L-Alanine powder with saturated L-alanine aqueous solution was used as a sample, which was compressed by a large-volume opposed-anvil assembly as presented in Fig. 1.1 Alanine (99.0% purity, Wako Pure Chemical Inds. Ltd) powder of 7 mg to 10 mg was loaded into a gasket and soaked in approximately 5 mg of alanine-saturated aqueous solution which was prepared using pure water (UV-sterilized Milli-Q water). The alanine-saturated solution was added as a pressure-transmitting medium. An encapsulating gasket of stainless steel (SUS304) was used to prevent the leakage of the liquid sample. Gaskets were cleaned in acetone ultrasonically for 5 min and were wiped using Kimwipes with pure water for 10 min while wearing laboratory gloves. The sample chamber volume was about 9.4 mm3.1 High-pressure experiments were conducted at room temperature (25 °C) on a CAPRICORN 500 ton press at the Institute for Solid State Physics, The University of Tokyo. The relationship between the load and generated pressure was obtained from the cell volume of NaCl, a standard pressure calibration material (Brown 1999), using a synchrotron X-ray facility (KEK, Photon Factory, AR-NE7).
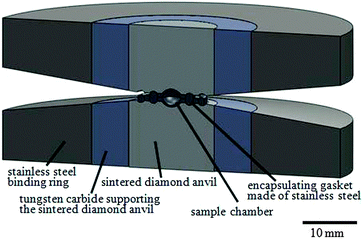 | ||
| Fig. 1 Double-toroidal anvil assembly. The center part was made of sintered diamond surrounded by tungsten carbide and a binding ring made of stainless steel. | ||
Four experimental runs were conducted with maximum pressures of approximately 5 GPa (25 ton), 7 GPa (40 ton), 9 GPa (60 ton), and 11 GPa (70 ton) with preservation times at maximum pressure for 1 h. Pressure was increased to maximum pressure for 15–30 min depending on the target pressure and was decreased to ambient pressure for the same duration after 1 hour preservation. Details of experimental parameters are presented in Table 1. After recovery, a gasket encapsulating alanine solution was stored immediately in 5 mL of pure water containing 4.19 μg of norvaline as standard for quantification.
| ND: not detected. | |||||
|---|---|---|---|---|---|
| Run no. | Standard | 1 | 2 | 3 | 4 |
| Load (ton) | — | 25 | 45 | 60 | 70 |
| Pressure (GPa) | — | 5 | 7 | 9 | 11 |
| Sat Ala aq. (mL) | — | 5 | 5 | 5 | 5 |
| Alanine (mg) | 6.43 | 9.82 | 6.99 | 7.85 | 10.16 |
| Alanylalanine (μg) | 0.11 | 0.89 | 1.19 | 4.62 | 20.22 |
| Alanylalanine (mmol mol−1) | 0.01 | 0.05 | 0.09 | 0.33 | 1.11 |
| Trialanine (μg) | ND | ND | ND | 0.18 | 2.28 |
| Trialanine (mmol mol−1) | ND | ND | ND | 0.01 | 0.13 |
Run products in the encapsulated gasket were extracted by breaking the gasket in the norvaline-containing aqueous solution used for the storage. Samples were analyzed using a previously reported procedure.12 The extracted sample solutions were dried and derivatized by 1.25 M HCl-isopropanol (Fluka Chemical), and then by trifluoroacetic anhydride (TFAA) (Tokyo Chemical Industry Co. Ltd) to produce N-TFA acylated-(Ala)n-O-isopropyl ester. The derivatized samples were identified and quantified using GC-MS (JMS-K9; JEOL Co.) equipped with a capillary column (0.25 mm i.d. × 30 m, 0.25 μm film thickness, HP-5; Agilent Technology Co.). The GC column temperature was operated as follows: maintained at 50 °C for 3 min, then increased to 225 °C at 3.5 °C min−1, followed by an increase to 325 °C at 5 °C min−1. It was finally held at 325 °C for 10 min, with He carrier gas (flux; 1 mL min−1). The ionization voltage of the mass spectrometer was set to 70 eV. The scan range of m/z was 40–550. Concentration of alanylalanine (alanine dimer) contained in the starting alanine reagent (99.0% purity, Wako Pure Chemical Inds. Ltd) was 0.01 mmol mol−1. That of trialanine (alanine trimer) was below the detection limit. Moreover, alanylalanine and trialanine were not detected from the procedure blank obtained from pure water loaded in the same gasket assembly with application of pressure of 5 GPa. Alanine peptides longer than tetramer, such as cycloalanylalanine, amines, and carboxylic acids, cannot be detected by the present experimental procedure and conditions.
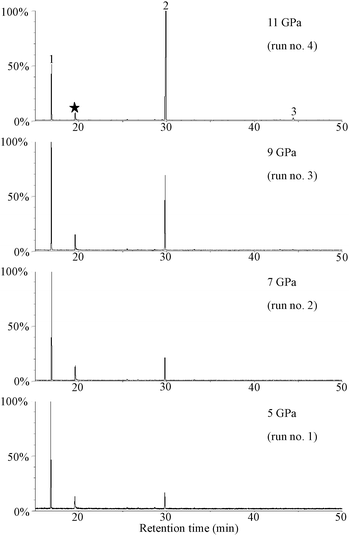
Fig. 2 shows mass chromatograms of m/z = 168 + 211 + 282 of samples recovered from 5 GPa, 7 GPa, 9 GPa, and 11 GPa. Each chromatogram is displayed by taking the most intense peak to be 100%. Numbered peaks 1, 2, and 3 correspond respectively to norvaline, alanylalanine, and trialanine. The oligomers (peak 2 and peak 3) were identified by comparison of their retention times and mass fragmental pattern with those of purchased standard materials. Fig. 3 presents a comparison of mass fragmental patterns of derivatives of alanylalanine and trialanine obtained from the run product of 11 GPa (run no. 4) with those of purchased standard materials.
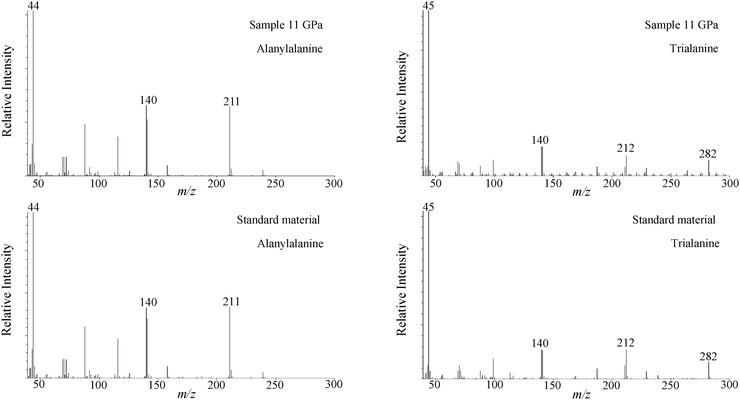 | ||
| Fig. 3 Representative mass fragmental patterns of derivatives of alanylalanine and trialanine recovered from 11 GPa, compared with those of standard materials. | ||
A solution containing known quantities of norvaline, alanylalanine, and trialanine was derivatized in the same way as the samples recovered from high pressure. The product of detection efficiency and yield of derivatization was calibrated from the peak areas corresponding to the derivatives of norvaline, alanylalanine, and trialanine in the mass chromatograms. Using these calibration data, quantities (weights) of alanylalanine and trialanine in the recovered samples were obtained. Concentrations of alanylalanine in all the run products were notably higher than the background concentration (0.01 mmol mol−1) in the starting material (see Table 1). In contrast, trialanine was detected in the two samples recovered from 9 GPa (run no. 3) and 11 GPa (run no. 4). Fig. 4 depicts molar ratios of alanylalanine and trialanine to starting alanine vs. pressure. With increasing pressure, yields of alanylalanine and trialanine increased. The maximum yield (0.11%) of alanylalanine was comparable to the oligomer yields described in reports of previous studies in terms of the order of magnitude.14
Reports of previous studies described that the formation of oligomers was promoted by energetically activating amino acid with vacuum ultraviolet irradiation, ion irradiation, catalysis, increasing temperature, and so on.15 In contrast, this study clarified that oligomerization of amino acids occurs at high pressure even at low temperatures without catalyst. It is noteworthy that the oligomerization of alanine occurs without application of additional activation at high pressure.
Solid alanine is in its zwitterion form. The N–H⋯O groups of the neighbouring molecules selectively approach each other with increasing pressure.11 The oligomerization with dehydration is regarded as occurring when the intermolecular distance of alanine molecules shortens with increasing pressure as well as that observed in the pressure-induced condensation of trimethylsilanol,9 in which most of the trimethylsilanol dimerized to form hexamethyldisiloxane accompanied with dehydration.9 In contrast, the respective maximum yields of alanylalanine and trialanine were 1.1 × 10−3 mol mol−1 and 1.3 × 10−4 mol mol−1 with oligomerization of alanine at 11 GPa in the present study.
The average intermolecular distance at 11 GPa should be longer than that at a reaction threshold, because the most of alanine molecules are unreacted and solid crystal structure of alanine is still remained at 11 GPa as reported in the diffraction studies.11 However, the intermolecular distance of neighbour alanine molecules fluctuates by molecular vibrations. Moreover, lattice defects or grain boundaries of alanine crystals could locally shorten intermolecular distances. Thus, the oligomerization might occur partially when the neighbour distance of the alanine molecules approaches a reaction threshold as well as that proposed in the pressure-induced oligomerization and amorphization of solid benzene.1,4,16 The hypothesis is supported by our results that the yields of the oligomers increase with increasing pressure as portrayed in Fig. 4.
Our results demonstrated that oligomerization of alanine occurred at pressures higher than 5 GPa even at 25 °C. The maximum yields of alanylalanine and trialanine were 1.1 × 10−3 and 1.3 × 10−4, respectively, at 11 GPa. This finding will open new windows for understanding the pressure-induced peptide formation from amino acids.
This work was supported by JSPS KAKENHI Grant Number 25287147 and 26246039. Part of this work was conducted under the Visiting Researcher's Program of the Institute for Solid State Physics, The University of Tokyo. The synchrotron radiation facility was used at NE7 of KEK, PF-AR with the approval of the Photon Factory Program Advisory Committee (Proposal No. 2013G609).
Notes and references
- A. Shinozaki, K. Mimura, H. Kagi, K. Komatu, N. Noguchi and H. Gotou, J. Chem. Phys., 2014, 141, 084306 CrossRef PubMed.
- T. C. Fitzgibbons, M. Guthrie, E. Xu, V. H. Crespi, S. K. Davidowski, G. D. Cody, N. Alem and J. V. Badding, Nat. Mater., 2015, 14, 43 CrossRef CAS PubMed.
- F. Cansell, D. Fabre and J. P. Petitet, J. Chem. Phys., 1993, 99, 7300 CrossRef CAS PubMed.
- L. Ciabini, M. Santoro, F. A. Gorelli, R. Bini, V. Schettino and S. Raugei, Nat. Mater., 2007, 6, 39 CrossRef CAS PubMed.
- L. Ciabini, M. Santoro, R. Bini and V. Schettino, Phys. Rev. Lett., 2002, 88, 085505 CrossRef.
- (a) M. Mugnai, G. Cardini and V. Schettino, J. Chem. Phys., 2004, 120, 5327 CrossRef CAS PubMed; (b) M. Citroni, M. Ceppatelli, R. Bini and V. Schettino, J. Chem. Phys., 2005, 123, 194510 CrossRef PubMed.
- (a) M. Mugnai, G. Cardini and V. Schettino, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 020101 CrossRef; (b) M. Citroni, M. Ceppatelli, R. Bini and V. Schettino, Science, 2002, 295, 2058 CrossRef CAS PubMed.
- (a) K. Aoki, S. Usuba, M. Yoshida, Y. Kakudate, K. Tanaka and S. Fujiwara, J. Chem. Phys., 1988, 89, 529 CrossRef CAS PubMed; (b) R. LeSar, J. Chem. Phys., 1987, 86, 1485 CrossRef CAS PubMed.
- A. Shinozaki, N. Noguchi and H. Kagi, Chem. Phys. Lett., 2013, 574, 66 CrossRef CAS PubMed.
- (a) S. A. Moggach, S. Parsons and P. A. Wood, Crystallogr. Rev., 2008, 14, 143 CrossRef CAS PubMed; (b) E. V. Boldyreva, Acta Crystallogr., 2008, A64, 218 CrossRef PubMed.
- (a) N. P. Funnell, A. Dawson, D. Francis, A. R. Lennie, W. G. Marshall, S. A. Moggach, J. E. Warren and S. Parsons, CrystEngComm, 2010, 12, 2573 RSC; (b) N. P. Funnel, W. G. Marshall and S. Parsons, CrystEngComm, 2011, 13, 5841 RSC.
- H. Sugahara and K. Mimura, Geochem. J., 2014, 48, 51 CrossRef CAS.
- S. Ohara, T. Kakegawa and H. Nakazawa, Origins Life Evol. Biospheres, 2007, 37, 215 CrossRef CAS PubMed.
- T. Otake, T. Taniguchi, Y. Furukawa, F. Kawamura, H. Nakazawa and T. Kakegawa, Astrobiology, 2011, 11, 8 CrossRef PubMed.
- (a) E. Imai, H. Honda, K. Hatori, A. Brack and K. Matsuno, Science, 1999, 283, 831 CrossRef CAS; (b) M. N. Islam, T. Kaneko and K. Kobayashi, Bull. Chem. Soc. Jpn., 2003, 76, 1171 CrossRef CAS; (c) M. B. Simakov, E. A. Kuzicheva, I. L. Mal'ko and N. Y. Dodonova, Adv. Space Res., 1996, 18, 61 CrossRef CAS; (d) M. Tanaka, F. Kaneko, T. Koketsu, K. Nakagawa and T. Yamada, Radiat. Phys. Chem., 2008, 77, 1164 CrossRef CAS PubMed; (e) M. B. Simakov, E. A. Kuzicheva, N. Y. Dodonova and A. E. Antropov, Adv. Space Res., 1997, 19, 1063 CrossRef CAS; (f) W. Wang, H. Yuan, X. Wang and Z. Yu, Adv. Space Res., 2007, 40, 1641 CrossRef CAS PubMed; (g) S. Fuchida, H. Masuda and K. Shinoda, Origins Life Evol. Biospheres, 2014, 44, 13 CrossRef CAS PubMed.
- S. Root and Y. M. Gupta, J. Phys. Chem. A, 2009, 113, 1268 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |