 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A family of low molecular-weight, organic catalysts for reductive C–C bond formation†
Saad
Shaaban
,
Anaïs
Jolit
,
Desislava
Petkova
and
Nuno
Maulide
*
University of Vienna, Institute of Organic Chemistry, Währinger Strasse 38, 1090 Vienna, Austria. E-mail: nuno.maulide@univie.ac.at; Web: http://maulide.univie.ac.at
First published on 21st July 2015
Abstract
Hydrazines form a new family of low molecular-weight reducing agents for diazonium salts. Using only small amounts of hydrazine catalyst, the coupling of diazonium salts to a variety of reactive partners has been achieved, without the requirement for either metal adjuvants or irradiation with visible or ultraviolet light. The generality of the concept proposed herein as well as its advantages in the preparative scale is outlined and discussed.
Diazonium salts are well-known highly reactive species in organic synthesis.1 Despite their commercially important application in the dye industry,2 diazonium salts function as ideal sources of organic radicals that engage in several important transformations such as the Meerwein arylation3 or the Sandmeyer4 and Pschorr reaction families.5 Since the advent6 and renaissance of photoredox catalysis,7 many photocatalysts have been shown to catalyse the single electron transfer reduction of diazonium salts to organic radicals. In particular, ruthenium(II) and iridium(III) complexes have been successfully applied in many reactions that exploit their remarkable reactivity in either reductive or oxidative quenching pathways upon visible light (440 nm) excitation.8 The relatively high price of those noble/precious metal complexes has subsequently led to the development of alternatives. Namely, several organic dyes proved to be efficient photocatalysts upon excitation with visible light.9 For instance, eosin Y,10 acridinium salts9a,11 and perylene diimides (PDI)12 were all successfully used in photocatalytic organic transformations. A recurrent structural feature of these organic dye catalysts is the presence of extended π-surfaces (mandated by the need to effectively absorb visible light) and high molecular-weight frameworks.13 Recently, Carrillo et al. described the use of ascorbic acid as an initiator for C–H arylation of anilines.14 Herein we describe a family of low-molecular weight organic catalysts that promote a range of C–C bond forming reactions of diazonium salts, and achieve so without the need for any metal adjuvant or irradiation with light.
We were inspired by the work of Shevlin et al.15 who aimed to isolate stable tetrazenes by reacting phenyldiazonium tetrafluoroborate with phenylhydrazine (Scheme 1). However, those authors were not able to isolate the tetrazene intermediate but instead observed a complex mixture that featured benzene as the major component in 54% yield and several other minor compounds. The formation of most of the components of the mixture was attributed to the intermediacy of free radicals, as supported by spectroscopic studies (CIDNP).
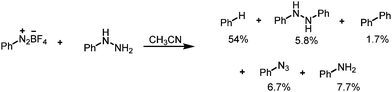 | ||
| Scheme 1 Reaction of the phenyldiazonium salt with phenylhydrazine as reported by Shevlin et al.15 | ||
Arylated heteroarenes have proved to be of interest for various applications, such as material sciences and biomedicine.16 One efficient route to install the aryl–heteroaryl bond is the Meerwein arylation, a cross-coupling reaction between heteroarenes and diazonium salts that is assumed to proceed by a free radical mechanism.17
We hypothesized that a hydrazine such as phenylhydrazine (employed in the work of Shevlin) could function as a novel reducing agent towards aryl diazonium salts. However, it was crucial to generate the radical intermediate under conditions which would (a) avoid the occurrence of either homocoupling or hydrogen abstraction (as seen by Shevlin), (b) enable its efficient trapping by the heteroarene partner, (c) prevent unwanted disproportionation reactions (such as, e.g. leading to an azide and an aniline, as originally also observed by Shevlin) and, most importantly, (d) allow rebound electron-transfer from the coupling adduct to generate the final, neutral biaryl product.
From the outset of our studies on the Meerwein arylation of thiophene, it soon became apparent that hydrazines might have the potential to function as catalysts in such coupling, thus addressing all the above concerns in a fell swoop (detailed optimisation is included as Table S1 of the ESI†). Strikingly, yields of coupling reactions were unchanged when the reaction was performed in the dark, showing that light plays no appreciable role in this transformation. We chose 4-aminomorpholine 3d to pursue our studies due to practical considerations and its low price. Importantly, the catalyst proved to be essential for the formation of 4a, as only trace amounts of the desired product were obtained under similar conditions if it was absent from the reaction mixture.18
The scope of this hydrazine-catalyzed C–H arylation of heteroarenes with various aryl diazonium salts was then examined using the best conditions found. As shown in Scheme 2, a range of functional groups, such as nitro, cyano, ester, and trifluoromethane, are compatible with the reaction conditions. Notably, the carbon–halogen bonds in 4g and 4i were not affected and could serve as reactive handles for further elaboration.19 A further potential advantage of this system is highlighted in Scheme 3. N,N-Dimethylhydrazine 3c, which has a very low molecular weight (MW = 60 g mol−1, more than 11 times lower than eosin Y), was used to scale up the coupling reaction between nitrobenzene diazonium salt 1a and furan 2b to yield 4b in ca. 85% yield in 15 min. Remarkably, only 7.6 mg of the catalyst was used for 12.6 mol of the substrate (3 g scale, 1 mol% catalyst). For the sake of comparison, to achieve the same reaction König's elegant photochemical method would require 87 mg of eosin Y (1 mol%).10b
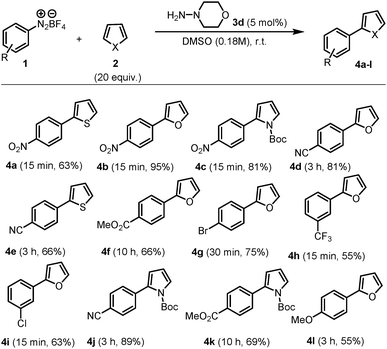 | ||
| Scheme 2 Scope of the α-arylation of heteroarenes with various diazonium salts using the optimised conditions detailed in the ESI.† The yields are given for the isolated products. | ||
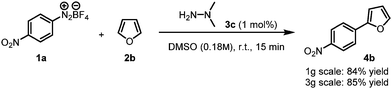 | ||
| Scheme 3 Scale up of the α-arylation of furan with the p-nitrobenzene diazonium salt using N,N-dimethylhydrazine 3c as a catalyst. | ||
At this juncture, we inquired whether this mode of redox catalysis could be extended to other related transformations. The synthesis of α-aryl ketones is a challenging significant reaction.20 It was recently shown that the capture of diazonium salts with enolacetates led to this motif.21 Nevertheless, the reported methodologies mandate the use of transition metal catalysts,21a transition metal photocatalysts21b or stoichiometric amounts of base.21c We thus were intrigued by the possibility of employing hydrazine catalysts for this process (Scheme 4).
 | ||
| Scheme 4 Preparation of α-aryl ketones via hydrazine-catalysed coupling of diazonium salts with alkenyl acetates 5. Yields refer to isolated products. | ||
Several aryl diazonium salts 1 were therefore treated with alkenyl acetates 5 in the presence of 10 mol% of hydrazine 3d in dimethylformamide (DMF) to prepare α-arylketone products 6. A series of α-aryl methylketones and α-aryl arylketones could be synthesized in moderate to good yield. The reaction was tolerant of several functional groups, such as nitro, ester and cyano substituents.
We further applied this catalytic system to the preparation of 2-substituted thiophenes 8 from the corresponding ortho-(methylthio) diazonium tetrafluoroborate 1h. This transformation has been previously reported using transition metal catalysis22a or visible light photoredox catalysis with eosin Y.22b 4-Aminomorpholine 3d was able to catalyse this transformation furnishing the corresponding benzothiophenes in good to moderate yields (Scheme 5).
 | ||
| Scheme 5 Preparation of 2-substituted benzothiophenes via hydrazine-catalysed coupling of diazonium salt 1h and alkynes 7. Yields refer to isolated products. | ||
Furthermore, the arylation of quinones with diazonium salts is an interesting transformation due to the important role of functionalized quinones in medicinal chemistry and biologically active compounds. Bearing this in mind, this reaction was also achieved employing 4-aminomorpholine 3d as a catalyst and the results are summarized in Scheme 6.23
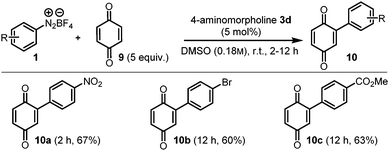 | ||
| Scheme 6 Hydrazine-catalysed C–H arylation of quinones with diazonium salts. Yields refer to isolated products. | ||
Finally, we attempted to trap the putative aryl radical intermediate, presumably formed upon diazonium salt reduction, with TEMPO 11. As shown in Scheme 7, phenyldiazonium salt 1g was exposed to a catalytic amount of 4-aminomorpholine 3d in the presence of 2 equiv. of TEMPO. The expected covalent adduct, 2,2,6,6-tetramethyl-1-phenoxypiperidine 12, was isolated in 44% yield. Additional experiments (see ESI† for details) reveal that the hydrazine catalyst is present in the reaction mixture (by both LC-MS and NMR analysis) after full conversion has been reached, thus establishing catalytic behaviour. Based on the above observations and previous literature,3b,10b,15 a general mechanism for the couplings of diazonium salts catalysed by 4-aminomorpholine (typified by the Meerwein arylation) is proposed in Scheme 8.
 | ||
| Scheme 8 Proposed catalytic cycle for the hydrazine-catalysed coupling of diazonium salt 13 and heteroarene 17. | ||
We believe that formation of tetrazene 14,15via reaction of diazonium salt 13 with the hydrazine catalyst 3d, will generate aryl radical 16 and diazenium radical cation 15 and is driven by the release of nitrogen gas. Radical 16 is subsequently intercepted by reactive unsaturation typified by 17, to form radical adduct 18. Oxidation of 18via SET of 1524 to the corresponding carbocation 19 regenerates the hydrazine catalyst 3d and aryl radical 16, enabling turnover.
In summary, we have shown that simple hydrazines form a new family of low molecular weight reducing agents that allow the catalytic coupling of diazonium salts to electron-rich heteroarenes, enol acetates, alkynes or benzoquinones under simple conditions. Notably, these catalysts' low molecular weight ensures unrivalled practicality when one considers the upscaling of this type of chemistry. The observation that hydrazines can function as catalytic activators for diazonium salts, shown by their presence after full conversion has been achieved, is a new type of reactivity bound to inspire the development of further transformations.
Generous financial support of this research by the Deutsche Forschungsgemeinschaft (Grants MA 4861/1-2 and MA 4861/4-2) and the University of Vienna is acknowledged.
Notes and references
- (a) H. Zollinger, Acc. Chem. Res., 1973, 6, 335–341 CrossRef CAS; (b) C. Galli, Chem. Rev., 1988, 88, 765–792 CrossRef CAS; (c) F. Minisci, F. Coppa, F. Fontana, G. Pianese and L. Zhao, J. Org. Chem., 1992, 57, 3929–3933 CrossRef CAS.
- (a) W. Dedichen, Chem. Ber., 1897, 30, 2657–2658 Search PubMed; (b) R. Loose, Chem. Ber., 1908, 41, 3906–3907 Search PubMed.
- (a) H. Meerwein, E. Buchner and K. Van Emsterk, J. Prakt. Chem., 1939, 152, 237 CrossRef CAS; (b) M. Heinrich, Chem. – Eur. J., 2009, 15, 820–833 CrossRef CAS PubMed.
- (a) T. Sandmeyer, Ber. Dtsch. Chem. Ges., 1884, 17, 1633 CrossRef , 2650; (b) E. Merkushev, Synthesis, 1988, 923–937 CrossRef CAS.
- (a) R. Pschorr, Ber. Dtsch. Chem. Ges., 1896, 29, 496–501 CrossRef CAS; (b) P. Leake, Chem. Rev., 1956, 56, 27–34 CrossRef CAS.
- (a) H. Cano-Yelo and A. Deronzier, J. Chem. Soc., Perkin Trans. 2, 1984, 1093–1098 RSC; (b) H. Cano-Yelo and A. Deronzier, Tetrahedron Lett., 1984, 25, 5517–5520 CrossRef CAS.
- For recent reviews on transition metal photocatalysis, see: (a) J. Narayanam and C. R. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (b) C. Prier, D. Rankic and D. W. C. MacMillan, ChemBioEng Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
- Selected references: (a) D. Nagib, M. Scott and D. W. C. MacMillan, J. Am. Chem. Soc., 2009, 131, 10875–10877 CrossRef CAS PubMed; (b) J. Nguyen, E. D'Amato, J. R. Narayanam and C. Stephenson, Nat. Chem., 2012, 4, 854–859 CrossRef CAS PubMed; (c) B. Sahoo, M. Hopkinson and F. Glorius, J. Am. Chem. Soc., 2013, 135, 5505–5508 CrossRef CAS PubMed; (d) X. Shu, M. Zhang, Y. He, H. Frei and F. Toste, J. Am. Chem. Soc., 2014, 136, 5844–5847 CrossRef CAS PubMed; (e) D. Hari, T. Hering and B. König, Angew. Chem., Int. Ed., 2014, 53, 725–728 CrossRef PubMed.
- For recent uses of organic dyes as photocatalysts, see: (a) S. Pitre, C. Mctiernan, H. Ismaili and J. Scaiano, J. Am. Chem. Soc., 2013, 135, 13286–13289 CrossRef CAS PubMed; (b) D. Nicewicz and T. Nguyen, ACS Catal., 2014, 4, 355–360 CrossRef CAS; (c) D. Hari and B. König, Chem. Commun., 2014, 50, 6688–6699 RSC.
- (a) A. Penzkofer, A. Beidoun and S. Speiser, Chem. Phys., 1993, 170, 139–148 CrossRef CAS; (b) D. Hari, P. Schroll and B. König, J. Am. Chem. Soc., 2012, 134, 2958–2961 CrossRef CAS PubMed; (c) M. Majeka and A. Wangelin, Chem. Commun., 2013, 49, 5507–5509 RSC; (d) J. Zhang, L. Wang, Q. Liu, Z. Yang and Y. Huang, Chem. Commun., 2013, 49, 11662–11664 RSC.
- (a) S. Fukuzumi, H. Kotani, K. Ohkubo, S. Ogo, N. Tkachenko and H. Lemmetyinen, J. Am. Chem. Soc., 2004, 126, 1600–1601 CrossRef CAS PubMed; (b) D. Hamilton and D. Nicewicz, J. Am. Chem. Soc., 2012, 134, 18577–18580 CrossRef CAS PubMed.
- (a) F. Würthner, Chem. Commun., 2004, 1564–1579 RSC; (b) I. Ghosh, T. Ghosh, J. Bardagi and B. König, Science, 2014, 346, 725–728 CrossRef CAS PubMed.
- Eosin Y Mw: 691.85 g mol−1, acridinium tetrafluoroborate Mw: 388.9 g mol−1, PDI Mw: 71031 g mol−1. For seminal work on organic superelectron donors, see: J. A. Murphy, J. Org. Chem., 2014, 79, 3731–3746 CrossRef CAS PubMed.
- For the use of ascorbic acid as a SET reductant (10 mol%) in the Meerwein arylation, see: F. P. Crisostomo, T. Martin and R. Carrillo, Angew. Chem., Int. Ed., 2014, 53, 2181–2185 CrossRef CAS PubMed.
- W. Evanochko and P. Shevlin, J. Am. Chem. Soc., 1979, 101, 4668–4672 CrossRef CAS.
- (a) J. Van Haare, L. Groenendaal, H. Peerlings, E. Havinga, J. Vekemans, R. Janssen and E. Meijer, Chem. Mater., 1995, 7, 1984–1989 CrossRef CAS; (b) B. Schmidt and D. Geißler, Eur. J. Org. Chem., 2011, 4814–4822 CAS.
- (a) B. Sreedhar, D. Yada and P. Reddy, Adv. Synth. Catal., 2011, 353, 2823–2836 CrossRef CAS; (b) N. Kuhl, M. Hopkinson and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 8230–8234 CrossRef CAS PubMed.
- For homolytic dediazoniation induced by solvent, see: G. Pratsch and M. Heinrich, in Radicals in Synthesis III, ed. M. Heinrich and A. Gansäuer, Springer, Berlin, 2012, vol. 320, pp. 33–59 Search PubMed.
- It is known that aryl diazonium salts bearing electron-withdrawing substituents give Meerwein products in higher yields: P. Caramella, G. Cellerino, A. C. Coda, A. G. Invernizzi, P. Grunanger, K. N. Houk and F. M. Albini, J. Org. Chem., 1976, 41, 3349–3356 CrossRef CAS.
- (a) T. Shen, Angew. Chem., 1972, 84, 512–526 CrossRef; (b) V. Aggarwal and B. Olofsson, Angew. Chem., Int. Ed., 2005, 44, 5516–5519 CrossRef CAS PubMed.
- (a) J. Rutherford, M. Rainka and S. Buchwald, J. Am. Chem. Soc., 2002, 124, 15168–15169 CrossRef CAS PubMed; (b) T. Hering, D. Hari and B. König, J. Org. Chem., 2012, 77, 10347–10352 CrossRef CAS PubMed; (c) C. Molinaro, J. Mowat, F. Gosselin, P. O'Shea, J. Marcoux, R. Angelaud and I. Davies, J. Org. Chem., 2007, 72, 1856–1858 CrossRef CAS PubMed.
- (a) R. Leardini, G. Pedulli, A. Tundo and G. Zanardi, J. Chem. Soc., Chem. Commun., 1985, 20, 1390–1391 RSC; (b) D. Hari, T. Hering and B. König, Org. Lett., 2012, 14, 5334–5337 CrossRef CAS PubMed.
- For arylation of quinones, see: (a) Y. Fujiwara, V. Domingo, I. Seiple, R. Gianatassio, M. Del Bel and P. Baran, J. Am. Chem. Soc., 2011, 133, 3292–3295 CrossRef CAS PubMed , base-mediated variant: ; (b) M. Lamblin, G. Naturalea, J. Dessolina and F.-X. Felpin, Synlett, 2012, 1621–1624 CrossRef CAS.
- The alternative reoxidation of 18 through SET from 13, as previously proposed in the literature (cf.ref. 10b and 14) cannot be ruled out at this stage.
Footnote |
| † Electronic supplementary information (ESI) available: Characterisation data for new compounds and ancillary experiments. See DOI: 10.1039/c5cc03580e |
| This journal is © The Royal Society of Chemistry 2015 |

