Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Quantitative determination of fluoride in pure water using luminescent europium complexes†
Stephen J.
Butler
Department of Chemistry, Loughborough University, Leistershire, LE11 3TU, UK. E-mail: s.j.butler@lboro.ac.uk
First published on 27th May 2015
Abstract
Two luminescent probes [Eu.L1–2]+ are reported for the rapid detection of fluoride in water. Probes [Eu.L1–2]+ exhibit exceptional enhancements in Eu emission in the presence of fluoride, permitting its selective determination within the environmentally relevant concentration range (20–210 μM).
Fluoride is considered essential for healthy teeth and bone growth; in several countries this has led to the artificial fluoridation of water supplies. Fluoride is also introduced into water supplies through the production of phosphate containing fertilizers and aluminium processing industries. Consumption of elevated levels of fluoride in drinking water can cause dental and skeletal fluorosis, as well as acute gastric problems and kidney failure. Therefore, controlling the level of fluoride in water supplies is a global governmental concern; the maximum recommended concentration of fluoride in drinking water defined by the World Health Organization guidelines is 4 mg L−1, or 210 μM. Consequently, the development of convenient analytical methods for the rapid, quantitative detection of fluoride in water samples has become an active area of research.
Traditional methods for the accurate analysis of fluoride involve the use of ion-selective electrodes and ion chromatography. However, such approaches can be time-consuming, require expensive instrumentation and are not practical for measuring fluoride concentrations in vivo. In recent years, a number of alternative strategies have been devised, such as the development of colorimetric or luminescent molecular probes, and reaction-based (irreversible) chemodosimeters.1–6 The creation of probes for fluoride recognition in water is particularly challenging because of the high free energy of hydration of fluoride 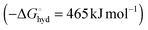 compared to other anions, together with its relatively small ionic radius (1.26 Å). Most probes reported to date have been limited in sensing applications, because they suffer from interference from other anions present in water samples (e.g. chloride, nitrate, sulfate and phosphate) that bind competitively. Furthermore, very few probes can actually detect fluoride ions in a pure aqueous medium;1 the majority of reported systems are restricted for use in organic solvents such as acetonitrile,2 DMSO,3 methanol4 or dichloromethane,5 or they require a solvent mixture of organic solvent and water.6 Reaction-based probes, such as those which rely on fluoride-mediated cleavage of a Si–O bond, are irreversible and typically suffer from delayed acquisition times (10 min to several hours).1b,d
compared to other anions, together with its relatively small ionic radius (1.26 Å). Most probes reported to date have been limited in sensing applications, because they suffer from interference from other anions present in water samples (e.g. chloride, nitrate, sulfate and phosphate) that bind competitively. Furthermore, very few probes can actually detect fluoride ions in a pure aqueous medium;1 the majority of reported systems are restricted for use in organic solvents such as acetonitrile,2 DMSO,3 methanol4 or dichloromethane,5 or they require a solvent mixture of organic solvent and water.6 Reaction-based probes, such as those which rely on fluoride-mediated cleavage of a Si–O bond, are irreversible and typically suffer from delayed acquisition times (10 min to several hours).1b,d
Over the last decade, several emissive lanthanide (Ln) complexes have been developed that can report changes in the concentrations of various anions, including citrate, lactate and bicarbonate, through modulation of emission spectral form or lifetime of the complex.7,8 However, Ln-based sensors for fluoride are scarce because the binding affinity between fluoride and Ln ions is generally too weak (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 1.5–3).8c,9 One notable example involved the encapsulation of fluoride between two Eu complexes, resulting in a supramolecular dimer (log
Ka = 1.5–3).8c,9 One notable example involved the encapsulation of fluoride between two Eu complexes, resulting in a supramolecular dimer (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = 13.0).1e In this case, the fluoride detection range was very low (0.2–50 μM), falling outside of the environmentally significant range (20–210 μM).
β = 13.0).1e In this case, the fluoride detection range was very low (0.2–50 μM), falling outside of the environmentally significant range (20–210 μM).
In this work, two water-soluble luminescent probes [Eu.L1–2]+ are reported (Fig. 1), each capable of binding and sensing fluoride in pure water samples, with minimal interference from other anions. Each probe is based on a C2-symmetric mono-cationic europium complex, bearing two trans-related quinoline chromophores and a coordinated water molecule. Fluoride binds reversibly to each probe, displacing the coordinated water, resulting in a 9-fold enhancement in the overall emission intensity and dramatic changes in emission spectral form (Fig. 2). Complex [Eu.L1]+ represents one of the very few probes capable of signalling the presence of fluoride within the range relevant to water fluoridation (20–210 μM),1a,d and provides an instantaneous spectral readout signal.
Details of the synthesis of complexes [Eu.L1–2]+ are provided in the ESI.† Selected photophysical data for [Eu.L1–2]+ are provided in Table 1. The UV-Vis absorption spectrum of [Eu.L1]+ is composed of a broad featureless band centred at 332 nm, whereas the absorption spectrum of [Eu.L2]+ features a narrow band with a maximum at 318 nm (Fig. S1 and S2, ESI†). The emission spectra of [Eu.L1–2]+ in water (25 mM HEPES, pH 7.4) were similar, each characterised by at least three components in the ΔJ = 1 transition (585–605 nm), indicating that both complexes adopt structures of low symmetry in water (Fig. 2a). In addition, the ΔJ = 2 transition (605–625 nm) is approximately equal in intensity to the ΔJ = 4 manifold (685–710 nm).
To assess the ability of [Eu.L1–2]+ to signal the presence of different anions, the emission spectrum of [Eu.L1]+ (20 μM) was recorded in water (25 mM HEPES, pH 7.4) in the presence of a 100-fold excess of a range of anions (Fig. 2). The addition of fluoride resulted in a 9-fold enhancement in the overall emission intensity of [Eu.L1]+, as well as substantial perturbations in the emission spectral form (Fig. 2b). The limiting spectrum indicated the formation of one major fluoride-bound species, as defined by a single ΔJ = 0 transition at 579 nm and two intense components within the ΔJ = 1 transition, centred at 586 and 596 nm respectively. In addition, the relative intensity of the hypersensitive ΔJ = 2 transition centred at 617 nm increased by a factor of 15. The only other anion that induced a significant spectral response was bicarbonate. In the presence of HCO3−, the limiting spectrum of [Eu.L1]+ (Fig. 2c) was distinctly different from that observed with fluoride. Most notably, a larger ratio between the transitions ΔJ = 2/ΔJ = 1 was observed. In contrast, all other anions typically present in water samples, including chloride, phosphate, sulfate and nitrate induced essentially no change (<10%) in emission intensity or spectral form (Fig. 2d). Complex [Eu.L2]+ also showed a selective spectral response to fluoride, giving rise to a smaller (3.5-fold) enhancement in the overall emission intensity (Fig. S6, ESI†).
Emission lifetimes of [Eu.L1]+ were measured in H2O and D2O in the presence of fluoride and were found to be similar (τ = 1.13 and 1.67 ms respectively), compared to those measured in the absence of a coordinating anion (τ = 0.49 and 1.38 ms). These results are consistent with a hydration state, q, of 1.2 (±20%) in the absence of fluoride, and zero for the fluoride-bound species.10 Thus, added fluoride results in displacement of the coordinated water molecule from the probe. The addition of HCO3− resulted in a similar change in the hydration state, whereas all other anions (e.g. Cl−, Br−, I−, HPO42−, CH3CO2−, HSO4−, NO3−) did not displace the bound water molecule. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode between [Eu.L1]+ and fluoride was confirmed by high resolution mass spectrometric data; a major signal was observed at m/z = 875.2302 for the singly charged ternary complex [151Eu.L1 + F + Na]+, in excellent agreement with the calculated isotopic distribution (Fig. S13, ESI†).
1 binding mode between [Eu.L1]+ and fluoride was confirmed by high resolution mass spectrometric data; a major signal was observed at m/z = 875.2302 for the singly charged ternary complex [151Eu.L1 + F + Na]+, in excellent agreement with the calculated isotopic distribution (Fig. S13, ESI†).
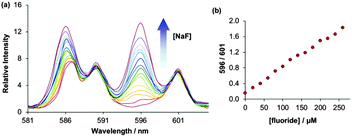
An affinity constant was determined for fluoride binding by making incremental additions of NaF to a solution of [Eu.L1]+ (20 μM) at pH 7.4 (25 mM HEPES). The change in the emission intensity ratio, 596/615 nm, was measured as a function of anion concentration, and the data were analysed using a non-linear least squares curve-fitting procedure based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (Fig. S4 and S5, ESI†). [Eu.L1]+ was found to bind to fluoride with log
1 binding model (Fig. S4 and S5, ESI†). [Eu.L1]+ was found to bind to fluoride with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 3.5 (±0.1). Under the same conditions, [Eu.L1]+ showed a slightly weaker affinity for bicarbonate [log
Ka = 3.5 (±0.1). Under the same conditions, [Eu.L1]+ showed a slightly weaker affinity for bicarbonate [log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 3.0 (±0.1)]. To eliminate competitive binding to HCO3− entirely, a fluoride titration experiment was conducted at pH 6 using 25 mM MES buffer (at pH 6, residual HCO3− is readily removed as CO2) (Fig. 3).11 Under these conditions, an affinity constant for the binding of fluoride to [Eu.L1]+ was log Ka = 4.1 (±0.1). This is 5 times stronger than that determined at pH 7.4, reflecting the absence of HCO3− at lower pH. Complex [Eu.L2]+, which lacks acetamide groups, showed slightly weaker binding to fluoride (log
Ka = 3.0 (±0.1)]. To eliminate competitive binding to HCO3− entirely, a fluoride titration experiment was conducted at pH 6 using 25 mM MES buffer (at pH 6, residual HCO3− is readily removed as CO2) (Fig. 3).11 Under these conditions, an affinity constant for the binding of fluoride to [Eu.L1]+ was log Ka = 4.1 (±0.1). This is 5 times stronger than that determined at pH 7.4, reflecting the absence of HCO3− at lower pH. Complex [Eu.L2]+, which lacks acetamide groups, showed slightly weaker binding to fluoride (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 3.5 ± 0.1) under the same conditions.
Ka = 3.5 ± 0.1) under the same conditions.
The affinity of [Eu.L1–2]+ for fluoride is substantially higher than that of previously reported Ln–F systems. This can be ascribed to a synergistic effect between Eu(III) coordination and additional hydrogen bonding interactions to the electron deficient quinoline units. A molecular model of [Eu.L2]+ with a coordinated fluoride ion [optimised at B3LYP/3-21G* with the Gaussian 09 package, using water as solvent]12 indicated that the bound fluoride can form two short C–H⋯F− contacts with each of the quinoline units (Fig. 4). The average C–H⋯F− distance is 2.81 Å, with an average H⋯F− distance of 1.88 Å. Both C–H⋯F− angles are acceptably linear (avg. angle is 141°).13 It is hypothesised that these bifurcated C–H⋯F⋯H–C contacts significantly stabilise the metal-bound fluoride. Such interactions would also restrict rotation of the quinoline N–Eu bonds, thus stabilising a single diastereomer, consistent with that observed in the Eu emission spectrum.
 | ||
| Fig. 4 An optimised structure of [Eu.L2]+ with a coordinated fluoride ion, stabilised by two short C–H⋯F− contacts (avg. C–H⋯F− distance is 2.81 Å, avg. C–H⋯F− angle is 141°). The model geometry was optimised at B3LYP/3-21G* (Gaussian 09 package) using the crystal structure of an Eu complex reported in ref. 14 as a starting point. | ||
The dramatic changes in Eu emission spectral form of [Eu.L1–2]+ observed in the presence of fluoride are indicative of alteration of the Eu(III) metal coordination environment. To probe this change further, the effect of fluoride on the 1H NMR spectrum of [Eu.L1]+ was investigated in D2O (pD 6.4). In the absence of added fluoride, two sets of proton resonances were clearly discernible in the 1H NMR spectrum of [Eu.L1]+, consistent with the presence of two diastereomers in solution (Fig. 5). The addition of 1 eq. of NaF resulted in the appearance of a new set of resonances corresponding to the fluoride-bound species, in slow exchange with the original hydrated complex on the NMR timescale. In the presence of excess NaF (5 eq.), the original signals disappeared and only signals for the fluoride-bound complex remained. The number of apparent signals has halved compared to the hydrated complex, indicating that a single fluoride-bound species has formed. Coordination of fluoride was also observed in the 19F NMR spectra, which showed a resonance at −123 ppm for unbound fluoride, and the emergence of a second signal at −474 ppm, corresponding to fluoride bound at the Eu metal centre (Fig. S11, ESI†).
 | ||
| Fig. 5 1H NMR spectra of [Eu.L1]+ (2.4 mM) upon addition of NaF (0–5 eq.). Measured in D2O (pD 6.4, 298 K). | ||
The remarkable sensitivity of the probe was demonstrated by plotting the emission intensity ratio, 596/601 nm, as a function of added NaF in the concentration range 0–260 μM (Fig. 6). The plot showed very good linearity (R2 = 0.9949) within this range. Notably, a 200% enhancement in the intensity ratio was obtained after the addition of 260 μM NaF. Competitive binding studies revealed that [Eu.L1–2]+ can detect low micromolar levels of fluoride with essentially no interference from other anions typically found in drinking water (e.g. Cl−, HPO42−, NO3− and SO42−), even when present in high concentrations (5 mM) (Fig. S9, ESI†). Thus, [Eu.L1]+ represents one of the most sensitive probes for the selective determination of fluoride levels in drinking water.
To validate the practicable utility of probe [Eu.L1]+, tap water samples buffered at pH 6 (25 mM MES) were spiked with known concentrations of NaF. The amount of fluoride was measured by comparing the recorded emission spectra with the calibration curve in Fig. 6 (Table 2). The results obtained using [Eu.L1]+ were in close agreement with the expected fluoride levels, with a maximal percentage variation of 7%. The results were also compared to those obtained independently using a fluoride-selective electrode (FSE); both analytical methods showed very good agreement.
| Spiked [F−]/μM | [F−]/μM | % Variationb | [F−]/μM |
|---|---|---|---|
Using [Eu.L1]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Using FSEa | ||
| a Values are the mean average of two independent measurements. b Calculated based on an initial F− ion concentration of 5 μM. | |||
| 30 | 36 (±6) | 3.0 | 35 (±3) |
| 65 | 65 (±4) | 7.0 | 68 (±3) |
| 115 | 113 (±4) | 6.0 | 111 (±4) |
| 160 | 154 (±6) | 6.5 | 165 (±4) |
| 210 | 204 (±8) | 5.0 | 212 (±4) |
In summary, two luminescent europium complexes [Eu.L1–2]+ have been synthesised, each capable of selective binding to fluoride in water in the 20–260 μM range. Such strong binding to fluoride can be attributed to a synergistic effect between Eu metal coordination and two stabilising C–H⋯F− interactions. [Eu.L1]+ was used for the quantitative determination of fluoride ions in drinking water samples, within the range relevant to water fluoridation (20–210 μM). These results establish Eu complexes as effective probes for fluoride analysis, offering advantages over traditional methods, including a rapid spectral readout signal. In addition, the ligand structure can be readily modified to modulate the steric demand at the Eu(III) metal centre, thus tuning anion affinity to the target concentration range.
SJB wishes to thank the Ramsay Memorial Fellowship Trust for funding, Dr Alan Kenwright and Dr Nicola Rodgers for help with NMR studies and Dr Mark Fox for molecular modelling studies.
References
- (a) R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915–4918 CrossRef CAS PubMed; (b) S. Y. Kim, J. Park, M. Koh, S. B. Park and J.-I. Hong, Chem. Commun., 2009, 4735–4737 RSC; (c) S. Rochat and K. Severin, Chem. Commun., 2011, 47, 4391–4393 RSC; (d) F. Zheng, F. Zeng, C. Yu, X. Hou and S. Wu, Chem. – Eur. J., 2013, 19, 936–942 CrossRef CAS PubMed; (e) T. Liu, A. Nonat, M. Beyler, M. Regueiro-Figueroa, K. Nchimi Nono, O. Jeannin, F. Camerel, F. Debaene, S. Cianférani-Sanglier, R. Tripier, C. Platas-Iglesias and L. J. Charbonnière, Angew. Chem., Int. Ed., 2014, 53, 7259–7263 CrossRef CAS PubMed.
- (a) J. F. Zhang, C. S. Lim, S. Bhuniya, B. R. Cho and J. S. Kim, Org. Lett., 2011, 13, 1190–1193 CrossRef CAS PubMed; (b) F. Han, Y. Bao, Z. Yang, T. M. Fyles, J. Zhao, X. Peng, Y. Wu and S. Sun, Chem. – Eur. J., 2007, 13, 2880–2892 CrossRef CAS PubMed; (c) M. Boiocchi, L. Del Boca, D. E. Gómez, L. Fabbrizzi, M. Licchelli and E. Monzani, J. Am. Chem. Soc., 2004, 126, 16507–16514 CrossRef CAS PubMed; (d) M. Vázquez, L. Fabbrizzi, A. Taglietti, R. M. Pedrido, A. M. González-Noya and M. R. Bermejo, Angew. Chem., Int. Ed., 2004, 43, 1962–1965 CrossRef PubMed.
- (a) S. Guha and S. Saha, J. Am. Chem. Soc., 2010, 132, 17674–17677 CrossRef CAS PubMed; (b) X. Cao, W. Lin, Q. Yu and J. Wang, Org. Lett., 2011, 13, 6098–6101 CrossRef CAS PubMed; (c) E. Quinlan, S. E. Matthews and T. Gunnlaugsson, J. Org. Chem., 2007, 72, 7497–7503 CrossRef CAS PubMed.
- S. J. M. Koskela, T. M. Fyles and T. D. James, Chem. Commun., 2005, 945–947 RSC.
- (a) X. Jiang, M. C. Vieweger, J. C. Bollinger, B. Dragnea and D. Lee, Org. Lett., 2007, 9, 3579–3582 CrossRef CAS PubMed; (b) C. R. Wade, I.-S. Ke and F. P. Gabbaï, Angew. Chem., 2012, 124, 493–496 CrossRef PubMed.
- (a) Y. Kim and F. P. Gabbaï, J. Am. Chem. Soc., 2009, 131, 3363–3369 CrossRef CAS PubMed; (b) P. Sokkalingam and C.-H. Lee, J. Org. Chem., 2011, 76, 3820–3828 CrossRef CAS PubMed; (c) T. Nishimura, S.-Y. Xu, Y.-B. Jiang, J. S. Fossey, K. Sakurai, S. D. Bull and T. D. James, Chem. Commun., 2013, 49, 478–480 RSC; (d) M. J. Langton, O. A. Blackburn, T. Lang, S. Faulkner and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11463–11466 CrossRef CAS PubMed.
- S. J. Butler and D. Parker, Chem. Soc. Rev., 2013, 42, 1652–1666 RSC.
- (a) R. Pal, D. Parker and L. C. Costello, Org. Biomol. Chem., 2009, 7, 1525–1528 RSC; (b) D. G. Smith, G.-l. Law, B. S. Murray, R. Pal, D. Parker and K.-L. Wong, Chem. Commun., 2011, 47, 7347–7349 RSC; (c) S. J. Butler, B. K. McMahon, R. Pal, D. Parker and J. W. Walton, Chem. – Eur. J., 2013, 19, 9511–9517 CrossRef CAS PubMed.
- L. S. M. P. Lima, A. Lecointre, J.-F. Morfin, A. de Blas, D. Visvikis, L. C. J. Charbonnière, C. Platas-Iglesias and R. Tripier, Inorg. Chem., 2011, 50, 12508–12521 CrossRef CAS PubMed.
- A. Beeby, I. M. Clarkson, R. S. Dickins, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. Gareth Williams and M. Woods, J. Chem. Soc., Perkin Trans. 2, 1999, 493–504 RSC.
- The concentration of HCO3− in water rises with increased pH. A pH titration revealed that the emission spectral profile of [Eu.L1]+ did not change at all within pH range 3.5–7.0 (where the level of HCO3− is negligible), but was sensitive to the increased amount of HCO3− present in the pH range 7.1–8.5 (Fig. S3, ESI†).
- Energy minimised structures were determined using the polarised continuum solvent model (PCM), using water as solvent.
- A search of the CCDC database reveals four examples of Ln complexes with a coordinated fluoride ion, and intramolecular C–H⋯F− contacts shorter than 3.1 Å. e.g. A. C. Hillier, X. Zhang, G. H. Maunder, S. Y. Liu, T. A. Eberspacher, M. V. Metz, R. McDonald, Â. Domingos, N. Marques, V. W. Day, A. Sella and J. Takats, Inorg. Chem., 2001, 40, 5106–5116 CrossRef CAS PubMed.
- S. Aime, A. S. Batsanov, M. Botta, J. A. K. Howard, M. P. Lowe and D. Parker, New J. Chem., 1999, 23, 669–670 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of complex synthesis and characterization, and UV-Vis, fluorescence and NMR spectral data are available. See DOI: 10.1039/c5cc03428k |
| This journal is © The Royal Society of Chemistry 2015 |