Open Access Article
Open Access ArticleTernary chalcogenide micro-pseudocapacitors for on-chip energy storage†
Narendra
Kurra‡
,
Chuan
Xia‡
,
M. N.
Hedhili
and
H. N.
Alshareef
*
Materials Science and Engineering, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: husam.alshareef@kaust.edu.sa
First published on 11th May 2015
Abstract
We report the successful fabrication of a micro-pseudocapacitor based on ternary nickel cobalt sulfide for the first time, with performance substantially exceeding that of previously reported micro-pseudocapacitors based on binary sulfides. The CoNi2S4 micro-pseudocapacitor exhibits a maximum energy density of 18.7 mW h cm−3 at a power density of 1163 mW cm−3, and opens up an avenue for exploring a new family of ternary oxide/sulfide based micro-pseudocapacitors.
Microsupercapacitors (MSCs) are promising devices for next generation on-chip energy storage due to their high power density, which can complement high energy density batteries.1–3 Additional attributes of MSCs include excellent rate capability, a long cycling lifetime, and superior volumetric capacity to their conventional sandwich counterparts.3,4 The planar configuration of MSCs can allow robust integration with functional electronic devices such as microsensors and biomedical implants as micro-power units.5–7 Hence, a great deal of research has been focused on a variety of nanostructured electroactive materials that are amenable to fabricate in-plane MSCs.3,4
Various MSCs based on conducting porous carbon were fabricated employing conventional photolithography and deposition or etching schemes. For example, high surface area carbon forms such as activated,8 carbide derived,2 onion like9 and carbon nanotubes10 have been patterned employing photolithography followed by deposition methods such as ink-jet printing, electrophoretic and spray deposition. Further, high temperature chemical vapor deposition (CVD) was also employed in growing carbon nanotubes (CNTs) and graphene or both, selectively over the patterned current collectors.11,12 Of late, due to compatible solution processability, graphene oxide (GO) has become attractive candidate material for making MSCs employing an etching scheme in a photolithography technique13 or innovative direct laser scribing methods.14,15 Further, in order to improve the limited capacitance of carbon based MSCs, several pseudocapacitive materials including metal oxides/hydroxides (RuO2, MnO2 and Ni(OH)2)16–18 and even conducting polymers (PPY, PANI, PEDOT)19–22 were employed to fabricate high capacity micro-pseudocapacitors. These pseudocapacitive materials can undergo fast redox reactions at their nanostructured surfaces, contributing to the higher values of capacity and hence energy density.16–22
Recently, new electrode materials in the form of binary and ternary sulfides have been explored as potential candidates for pseudocapacitors.23–25 However, most of the studies have been focused on their synthesis and investigation of their electrochemical performance in 3-electrode configuration. To exploit the practical usage of these new materials, it is highly desirable to build energy storage devices in a 2-electrode configuration.25,26 In-plane MSCs based on binary metal sulfides such as MoS2 and VS2 have been fabricated, which showed an areal cell capacitance of 5–8 mF cm−2.27,28 Moreover, nanostructured sulfides, in particular, ternary nickel cobalt sulfides, have become outstanding electrode materials due to their higher conductivity and richer redox reactions than the single component sulfides due to contributions from nickel and cobalt ions.23–25 In addition, it was demonstrated that these ternary cobalt nickel sulfides exhibit higher electronic conductivity and richer redox reactions than their ternary oxide counter-parts, resulting in higher capacity values.29 Here, we demonstrate the fabrication of ternary sulfide micro-pseudocapacitors with excellent volumetric capacitance and energy density.
We report the fabrication of a nanostructured ternary cobalt nickel sulfide micro-pseudocapacitor employing electrochemical co-deposition over the rGO/Au/patterned photoresist. The rGO free-standing films are prepared through vacuum filtration from its dispersion. Before doing lift-off, ternary cobalt nickel sulfide was electrodeposited to ensure uniform deposition over the entire area. The resulting CoNi2S4 MSC exhibits a volumetric capacitance of 211 F cm−3 (an areal capacitance of 11.6 mF cm−2), corresponding to an energy density of 18.7 mW h cm−3 at a power density of 1163 mW cm−3. This is the best reported performance of any sulfide based micro-pseudocapacitor.
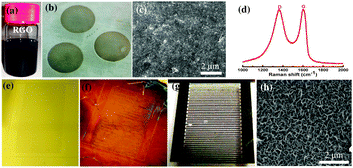
Fig. 1a shows the rGO dispersion (described in the ESI†) that was used to obtain rGO thin films by vacuum filtration over the anodic alumina membrane.30–32 The alumina membrane was subsequently dissolved in 3 M NaOH to result in the freely floating film of rGO over the water bath as shown in Fig. 1b. This rGO film is comprised of tightly packed small flakes (lateral dimensions, 200 nm) giving rise to innumerable edges, domain boundaries, and interfaces. Raman spectrum of rGO film shows broad D and G bands centered at 1367 (FWHM, 206 cm−1) and 1598 cm−1 (FWHM, 91 cm−1), respectively. The D band originates from the presence of defects and functional groups while the G band corresponds to symmetric stretching of the sp2 carbon lattice with E2g symmetry.33 The ID/IG ratio of rGO films was found to be in the range of 0.8–0.9 and the sp2 crystallite size was estimated using the below formula,34 and was found to be 10–15 nm. Elaser is the laser excitation energy (eV) in the following equation:
| La (nm) = 560/Elaser4(ID/IG)−1 | (1) |
We have followed the conventional photolithography lift-off protocol to fabricate ternary nickel cobalt sulfide micro-pseudocapacitors (the detailed fabrication process is illustrated in the ESI,† Fig. S1). Initially, a metal coated patterned photoresist chip was employed as a platform to transfer the free-standing rGO film as shown in Fig. 1e and f. Reduced graphene oxide (rGO) provides a functional conducting graphitic platform which, when used as current collector, improves the nucleation and growth of pseudocapacitive materials compared to metal current collectors.35 Recently, Du et al. have demonstrated a physical approach for synthesizing a nanocomposite of CoNi2S4–graphene with excellent electrochemical performance.36 We have found that compared to metal surfaces such as Au or Ni, the functional rGO surface helps in the uniform growth of ternary sulfides (Fig. S2, ESI†). It was observed that the growth of CoNi2S4 was non-uniform over the Au current collectors as is evident from the SEM micrographs shown in Fig. S2 (ESI†). However, Au acts as a conductive support/current collector while the rGO layer helps in the homogenous and uniform nucleation and growth of CoNi2S4 during the electrochemical co-deposition process. Electrochemical co-deposition was carried out before photoresist lift-off to ensure uniform electric field and uniform growth of CoNi2S4 over the entire chip. Doing the photoresist lift-off before electrodeposition would have created irregular electric fields and non-uniform growth of the CoNi2S4. The strong adherence of nickel cobalt sulfide onto the rGO film is evident since the final step of lift-off involved rigorous ultrasonication to make the CoNi2S4/rGO finger electrodes. It is obvious from the SEM micrograph shown in Fig. 1h that the electrodeposited CoNi2S4 layer has a uniform monolithic morphology. We have considered rGO thin film as a platform for growing CoNi2S4 heterostructures with interdigitated electrode patterns in evaluating its electrochemical performance in a 2-electrode configuration.
XPS analysis was carried out in order to determine the chemical composition of the sample and the oxidation state of detected elements. High resolution XPS spectra of Co 2p, Ni 2p, and S 2p core levels have been recorded and are shown in Fig. 2. The high resolution XPS spectra match well with those obtained for ternary nickel cobalt sulfides CoNi2S437 and NiCo2S4.38,39 Precise distinction between the two sulfides is difficult due to the similarities of the shape and binding energies of the Co 2p, Ni 2p and S 2p core levels. The elemental composition was obtained using XPS by applying the appropriate relative sensitivity factors (RSFs) to the integrated peak areas of specific core levels for the different elements. The atomic ratio S/Ni/Co was estimated to be 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which can be assigned to CoNi2S4. The Ni 2p3/2 portion of the Ni 2p spectrum was fitted using three components.
1, which can be assigned to CoNi2S4. The Ni 2p3/2 portion of the Ni 2p spectrum was fitted using three components.
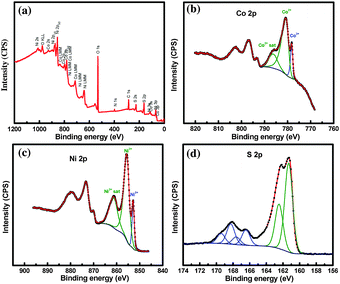 | ||
| Fig. 2 X-ray photoelectron spectroscopy (XPS) of CoNi2S4 (a) survey scan and core-level spectra of (b) Co 2p, (c) Ni 2p and (d) S 2p. | ||
The Ni 2p3/2 components observed at 852.9 and 855.7 eV correspond to Ni2+ and Ni3+ species, respectively.37,40 The third Ni 2p3/2 component located at 861.3 eV is related to a shake-up satellite of Ni3+ species.37,40 The Co 2p3/2 part of the Co 2p spectrum was fitted using three components as well. The Co 2p3/2 component observed at 778.2 is attributed to Co3+ species, while the components located at 781.0, and 786. 3 eV correspond to the Co2+ main peak accompanied with its shake-up satellite, respectively.37,40 For the S 2p high resolution XPS spectrum, the peak observed at 161.3 eV corresponds to the S 2p3/2 core level, which is typical of metal sulfur bounds in the ternary metal sulfides41 and consistent with nickel and cobalt sulfides.41 The S 2p3/2 peaks observed at 166.5 eV and 168.3 eV are attributed to surface sulfur with high oxide states, to sulfites and sulfates, respectively.
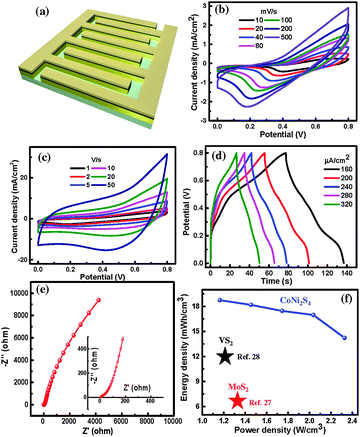
The electrochemical performance of the CoNi2S4 micro-pseudocapacitor was tested in a 2-electrode configuration in 1 M KOH electrolyte. Unlike the EDLC behavior of an rGO MSC where the charge is stored through the electrostatic accumulation of electrolytic ions, redox behavior is observed from the CoNi2S4 MSC (see Fig. S3, ESI†). As shown in Fig. 3a, the redox peaks appear at different scan rates due to the redox behavior of Co+4/Co+3/Co+2 and Ni+3/Ni+2 couples, mediated by the hydroxyl ions (OH−) in the electrolyte. Since sulfur belongs to the same group of oxygen, OH− ions can induce electrochemical oxidation and reduction of CoNi2S4 in alkaline medium similar to that of metal oxides/hydroxides. However, it is difficult to deconvolute the contributions of Ni and Co due to the broad nature of redox peaks, most probably due to reversible formation of redox couples such as NiS/NiS(OH) and CoS/CoS(OH)/CoSO. At a scan rate of 10 mV s−1, CoNi2S4 shows an areal cell capacitance of 11.6 mF cm−2, which is superior to binary metal oxide (areal cell capacitance of 1–5 mF cm−2) and sulfide (VS2, MoS2; CA = 4–8 mF cm−2) based micro-pseudocapacitors. It was found that the areal capacitance was gradually increasing from 1.7 to 11.6 mF cm−2 (at a current density of 160 μA cm−2) due to more loading of CoNi2S4 when the deposition cycles were increased from 1 to 6 (see Fig. S4, ESI†). Due to the planar configuration of the CoNi2S4 interdigitated electrodes, CVs were recorded even at higher scan rates of 1–50 V s−1 as shown in Fig. 3c. Similarly, charge–discharge curves of the CoNi2S4 MSC at different current densities were recoded as shown in Fig. 3d. CD profiles are highly non-linear, clearly demonstrating the pseudocapacitive behavior of the CoNi2S4 electrodes. We have found that this micro-pseudocapacitor exhibits an areal cell capacitance of 11.6 mF cm−2 (thickness of CoNi2S4/rGO is about 0.55 μm), which corresponds to a volumetric capacitance of 211 F cm−3, while the pristine rGO based MSC shows a much lower areal capacitance of 0.3 mF cm−2 with a volumetric capacitance of 40 F cm−3. Cycling stability of the CoNi2S4 MSC was tested by continuous charging–discharging at a current density of 320 μA cm−2, which showed a capacitance retention of 80% after 5000 cycles with a coulombic efficiency of above 95% (see Fig. S5, ESI†). Further, a solid state CoNi2S4 MSC was fabricated by employing PVA/KOH gel electrolyte, which exhibits an areal capacitance of 2.8 mF cm−2 (see Fig. S6, ESI†). Thus, enhanced values of areal and volumetric capacitance of the CoNi2S4 MSC are due to the pseudocapacitive nature unlike the EDLC behavior of carbon based MSCs. This volumetric capacitance is still higher than MoS2 MSC (178 F cm−3). Thus, these ternary CoNi2S4 with impressive redox contributions from the Ni and Co metals as well as facile ionic diffusion through the nanostructured electrodes give rise to higher volumetric capacitance. The impedance spectrum for the CoNi2S4 micro-pseudocapacitor is shown in Fig. 3e. The presence of a semi-circle in the high frequency region is due to charge-transfer resistance across the electrode/electrolyte interface with an equivalent series resistance (ESR) of 5 Ω (see the inset of Fig. 3e). An optimized CoNi2S4 micro-pseudocapacitor exhibits a maximum energy density of 18.7 mW h cm−3 at a power density of 1163 mW cm−3. The energy density values are at least twice that of carbon8–15 and metal oxide based MSCs16,17 (E = 1–10 mW h cm−3) fabricated in the literature. As shown in the Ragone plot in Fig. 3f, the CoNi2S4 MSC exhibits superior energy density over the binary sulfide based MSCs such as MoS2 (E = 6 mW h cm−3)27 and VS2 (E = 15 mW h cm−3) (see Table S1, ESI†).28 The CoNi2S4 MSC exhibits a maximum power density of 2327 mW cm−3 even at an energy density of 14.2 mW h cm−3. This study opens up the possibility of employing ternary sulfides as potential candidate electrode materials for on-chip energy storage with superior volumetric capacitance and energy density.
In summary, we have demonstrated the successful fabrication of a micro-pseudocapacitor based on ternary nickel cobalt sulfide. This was achieved by employing single-step electrochemical co-deposition of nickel cobalt sulfide over transferred reduced graphene oxide (rGO) thin films followed by photoresist lift-off. The fabricated CoNi2S4 micro-pseudocapacitor exhibits a maximum areal cell capacitance of 11.6 mF cm−2 with a volumetric capacitance of 211 F cm−3. The maximum energy density of 18.7 mW h cm−3 at a power density of 1163 mW cm−3 was achieved, which is superior to the binary sulfide micro-supercapacitors reported in the literature. This study may open up an opportunity to fabricate various kinds of ternary oxide/sulfide based micro-pseudocapacitors.
Research reported in this publication was supported by King Abdullah University of Science and Technology (KAUST). The authors thank the Advanced Nanofabrication, Imaging and Characterization Laboratory at KAUST for their excellent support.
Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- J. Chmiola, C. Largeot, P. L. Taberna, P. Simon and Y. Gogotsi, Science, 2010, 328, 480 CrossRef CAS PubMed.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867 CAS.
- G. Xiong, C. Meng, R. G. Reifenberger, P. P. Irazoqui and T. S. Fisher, Electroanalysis, 2014, 26, 30 CrossRef CAS PubMed.
- F. Simjee, P. H. Chou, Everlast: Long-life, Supercapacitor-operated Wireless Sensor Node, Low Power Electronics and Design, 2006. ISLPED′06. Proceedings of the 2006 International Symposium on, 4–6 Oct. 2006, pp. 197–202.
- Z. L. Wang, Adv. Mater., 2012, 24, 280–285 CrossRef CAS PubMed.
- Z. L. Wang and W. Wu, Angew. Chem., Int. Ed., 2012, 51, 11700–11721 CrossRef CAS PubMed.
- D. Pech, M. Brunet, P. L. Taberna, P. Simon, N. Fabre, F. Mesnilgrente, V. Cońed'era and H. Durou, J. Power Sources, 2010, 195, 1266 CrossRef CAS PubMed.
- D. Pech, M. Brunet, H. Durou, P. Huang, V. Mochalin, Y. Gogotsi, P.-L. Taberna and P. Simon, Nat. Nanotechnol., 2010, 5, 651 CrossRef CAS PubMed.
- M. Beidaghi and C. L. Wang, Adv. Funct. Mater., 2012, 22, 4501 CrossRef CAS PubMed.
- J. Lin, C. Zhang, Z. Yan, Y. Zhu, Z. Peng, R. H. Hauge, D. Natelson and J. M. Tour, Nano Lett., 2012, 13, 72 CrossRef PubMed.
- G. Xiong, C. Meng, R. G. Reifenberger, P. P. Irazoqui and T. S. Fisher, Energy Technol., 2014, 2, 897 CrossRef CAS PubMed.
- Z. S. Wu, K. Parvez, X. Feng and K. Müllen, Nat. Commun., 2013, 4, 2487 Search PubMed.
- W. Gao, N. Singh, L. Song, Z. Liu, A. L. M. Reddy, L. Ci, R. Vajtai, Q. Zhang, B. Wei and P. M. Ajayan, Nat. Nanotechnol., 2011, 6, 6 CrossRef PubMed.
- M. F. El-Kady and R. B. Kaner, Nat. Commun., 2013, 4, 1475 CrossRef PubMed.
- T. M. Dinh, K. Armstrong, D. Guay and D. Pech, J. Mater. Chem. A, 2014, 2, 7170 CAS.
- W. Si, C. Yan, Y. Chen, S. Oswald, L. Han and O. G. Schmidt, Energy Environ. Sci., 2013, 6, 3218 CAS.
- N. Kurra, N. A. Alhebshi and H. N. Alshareef, Adv. Energy Mater., 2014, 4, 1401303 Search PubMed.
- M. Beidaghi and C. L. Wang, Electrochim. Acta, 2011, 56, 9508 CrossRef CAS PubMed.
- K. Wang, W. Zou, B. Quan, A. Yu, H. Wu, P. Jiang and Z. Wei, Adv. Energy Mater., 2011, 1, 1068 CrossRef CAS PubMed.
- C. Meng, J. Maeng, S. W. M. John and P. P. Irazoqui, Adv. Energy Mater., 2014, 4, 1301269 Search PubMed.
- N. Kurra, M. K. Hota and H. N. Alshareef, Nano Energy, 2015, 13, 500 CrossRef CAS PubMed.
- H. Wan, J. Jiang, J. Yu, K. Xu, L. Miao, L. Zhang, H. Chen and Y. Ruan, CrystEngComm, 2013, 15, 7649 RSC.
- S. Peng, L. Li, C. Li, H. Tan, R. Cai, H. Yu, S. Mhaisalkar, M. Srinivasan, S. Ramakrishna and Q. Yan, Chem. Commun., 2013, 49, 10178 RSC.
- W. Chen, C. Xia and H. N. Alshareef, ACS Nano, 2014, 8, 9531 CrossRef CAS PubMed.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294 CAS.
- L. Cao, S. Yang, W. Gao, Z. Liu, Y. Gong, L. Ma, G. Shi, S. Lei, Y. Zhang, S. Zhang, R. Vajtai and P. M. Ajayan, Small, 2013, 9, 2905 CrossRef CAS PubMed.
- J. Feng, X. Sun, C. Wu, L. Peng, C. Lin, S. Hu, J. Yang and Y. Xie, J. Am. Chem. Soc., 2011, 133, 17832 CrossRef CAS PubMed.
- J. Xiao, X. Zeng, W. Chen, F. Xiao and S. Wang, Chem. Commun., 2013, 49, 11734–11736 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101 CrossRef CAS PubMed.
- M. Shahid, N. Yesibolati, F. M. Ross and H. N. Alshareef, J. Power Sources, 2014, 263, 239 CrossRef CAS PubMed.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095 CrossRef CAS.
- M. A. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. G. Cançado, A. Jorioa and R. Saito, Phys. Chem. Chem. Phys., 2007, 9, 1276 RSC.
- Z.-S. Wu, G. Zhou, L.-C. Yin, W. Ren, F. Li and H.-M. Cheng, Nano Energy, 2012, 1, 107 CrossRef CAS PubMed.
- W. Du, Z. Wang, Z. Zhu, S. Hu, X. Zhu, Y. Shi, H. Pang and X. Qian, J. Mater. Chem. A, 2014, 2, 9613 CAS.
- W. Du, Z. Wang, Z. Zhu, S. Hu, X. Zhu, Y. Shi, H. Pang and X. Qian, J. Mater. Chem. A, 2014, 2, 9613 CAS.
- J. Xiao, X. Zheng, W. Chen, F. Xiao and S. Wang, Chem. Commun., 2013, 49, 11734 RSC.
- J. Xiao, L. Wan, S. Yang, F. Xiao and S. Wang, Nano Lett., 2014, 14, 831 CrossRef CAS PubMed.
- M. Prabu, K. Keptpang and S. Shanmugam, Nanoscale, 2014, 6, 3173 RSC.
- Y. Ohno, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 1281 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc03220b |
| ‡ Contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2015 |