Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organoaqueous calcium chloride electrolytes for capacitive charge storage in carbon nanotubes at sub-zero-temperatures†
Yun
Gao
ab,
Zhanbin
Qin
b,
Li
Guan
a,
Xiaomian
Wang
b and
George Z.
Chen
 *ac
*ac
aDepartment of Chemical and Environmental Engineering, and Energy and Sustainability Research Division, Faculty of Engineering, The University of Nottingham, Nottingham NG7 2RD, UK. E-mail: george.chen@nottingham.ac.uk
bDepartment of Chemical Engineering, North China University of Science and Technology, Tangshan, Hebei 063009, P. R. China
cDepartment of Chemical and Environmental Engineering, and Centre for Sustainable Energy Technologies, Faculty of Science and Engineering, The University of Nottingham Ningbo China, Ningbo 315100, P. R. China
First published on 26th May 2015
Abstract
Solutions of calcium chloride in mixed water and formamide are excellent electrolytes for capacitive charge storage in partially oxidised carbon nanotubes at unprecedented sub-zero-temperatures (e.g. 67% capacitance retention at −60 °C).
Electrochemical capacitors (ECs), also known as supercapacitors, are fast emerging energy storage devices.1 Current EC research is mainly focused on new electrode materials,2 but electrolytes are also an essential factor deserving more attention. Electrolytes in ECs can be broadly classified into non-aqueous and aqueous. Non-aqueous electrolytes3 are based on either organic solvents, such as propylene carbonate, acetonitrile, or ionic liquids (ILs). Organic electrolytes can offer wide potential windows that are needed for high energy storage capacity, but also have high volatility and low flaming points, causing safety concerns. ILs are much less volatile but they are relatively expensive and also too viscous to offer good conductivity. Aqueous electrolytes4 are usually concentrated solutions of salts, acids or bases. They are restricted by narrow potential windows due to decomposition of water, but have many advantages over non-aqueous electrolytes, such as high conductivity, affordable cost, and low toxicity. These merits of water based electrolytes make them particularly attractive for large scale and stationary applications such as storage of electricity generated from wind turbine farms.
Nevertheless, it is still a challenge to use aqueous electrolytes. Water freezes at 0 °C, while winds are often stronger and more frequent in places where cold winter often experiences sub-zero temperatures. Hence, for low temperature applications, the electrolytes used in supercapacitors (and batteries) are commonly non-aqueous,5 whilst aqueous electrolytes are rarely considered.6 However, the very recent finding of a specific capacitance value of 30 F g−1 is encouraging by testing the MnO2–carbon composites in mixed water, ethylene glycol and (NH4)2SO4 at −30 °C.6a
It is known that mixing two liquids may lead to eutectic behaviour if hydrogen bonds are involved.7 In this work, a simple mixture of formamide (FA) and water (FA–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) was found to start and finish freezing between −32 °C (liquidus temperature) and −44 °C (solidus temperature). This liquidus–solidus temperature range (LSTR) is much lower than the melting point (m.p.) of FA (2 °C) or H2O. This observation can be explained as follows. First, the vapor pressure of any component in a mixture is lower than that of the component alone from the viewpoint of physical evaporation, leading to a LSTR lower than the m.p. of each component. More importantly, FA and H2O are both hydrogen bond donors and acceptors. As a result, hydrogen bonds are plentiful in the FA–H2O mixture and can contribute to lowering the LSTR. (Note: we cannot find the FA–H2O binary phase diagram in the literature and therefore constructed it in this work as presented in Fig. S1 in the ESI.†)
v) was found to start and finish freezing between −32 °C (liquidus temperature) and −44 °C (solidus temperature). This liquidus–solidus temperature range (LSTR) is much lower than the melting point (m.p.) of FA (2 °C) or H2O. This observation can be explained as follows. First, the vapor pressure of any component in a mixture is lower than that of the component alone from the viewpoint of physical evaporation, leading to a LSTR lower than the m.p. of each component. More importantly, FA and H2O are both hydrogen bond donors and acceptors. As a result, hydrogen bonds are plentiful in the FA–H2O mixture and can contribute to lowering the LSTR. (Note: we cannot find the FA–H2O binary phase diagram in the literature and therefore constructed it in this work as presented in Fig. S1 in the ESI.†)
The low LSTR of the FA–H2O mixture promoted us to use it to dissolve CaCl2 for the preparation of low-temperature electrolytes. CaCl2 was selected for several reasons. Although not used in past supercapacitor research, CaCl2 is a low cost salt with its toxicity (as animal food additive) and water solubility comparable to those of NaCl. Also, for road de-icing in winter, CaCl2 is widely used mainly because it gives a eutectic temperature of −51.6 °C, whilst NaCl could only reach −21.1 °C.6c This ability of CaCl2 results possibly from the high affinity between calcium and oxygen. In thermodynamic terms, CaO is the most stable metal oxide next to CeO2. This high calcium–oxygen affinity can be translated to that between Ca2+ ions and the oxygen atoms in water molecules. For the same reason, CaCl2 is extremely hygroscopic and often used as a drying agent in laboratory.
We believe that this unique nature of CaCl2 can make it a better electrolyte than other common salts, such as KCl and Na2SO4, for capacitive charge storage on carbon surfaces enriched with e.g. carbonyl, carboxyl and hydroxyl groups or simply oxy-groups. In line with this thought, carbon nanotubes (CNTs) were selected for electrochemical tests because CNTs are well-known, particularly after partial oxidation in acids, to possess surface oxy-groups.8 The affinity of Ca2+ ions to oxy-groups explains the unique Ca2+ selectivity of the ionophore A23187 over alkali metal ions (see ESI†). Furthermore, a pure carbon surface is hydrophobic, but because of the oxy-groups, the CNT surfaces are amphiphilic in nature,8c and may be better accessed by an organoaqueous electrolyte.
Three CaCl2 solutions were prepared using H2O, FA, or FA–H2O (vol ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which was selected to compromise CaCl2 solubility, viscosity and liquid temperature range). As expected, CaCl2 addition lowered the liquidus temperature to −16 °C for CaCl2–H2O (2.0 mol L−1), −41 °C for CaCl2–FA (1.0 mol L−1), and below −60 °C for CaCl2–FA–H2O, (2.0 mol L−1), respectively.
1 which was selected to compromise CaCl2 solubility, viscosity and liquid temperature range). As expected, CaCl2 addition lowered the liquidus temperature to −16 °C for CaCl2–H2O (2.0 mol L−1), −41 °C for CaCl2–FA (1.0 mol L−1), and below −60 °C for CaCl2–FA–H2O, (2.0 mol L−1), respectively.
To prepare the CNT working electrode, 20 μL aqueous suspension of acid treated CNTs8 with PTFE (binder, 5 wt% after drying) was dropped on a 5 mm diameter graphite disc electrode, giving rise to a CNT loading of ca. 0.2 mg cm−2. After drying in air (>5 h), the CNT electrode was used to record cyclic voltammograms (CVs) in different electrolytes. A graphite rod and the Ag/AgCl couple were used as the counter and reference electrodes, respectively (see ESI† for more details of the three electrode cell).
In this work, temperature control was achieved in two ways. To determine the liquidus and solidus temperatures, an ultralow temperature thermostat bath was used. For cyclic voltammetry, the cell containing 10 mL of the electrolyte was placed in a thermo-container filled with dry ice (−78.5 °C). A thermocouple was inserted in the electrolyte to monitor the temperature. It was observed that the electrolyte temperature dropped fairly slowly, e.g. <1 °C min−1 below −55 °C, to enable several CV cycles at a designated temperature and a sufficiently high potential scan rate, e.g. 100 mV s−1 (20 s per cycle).
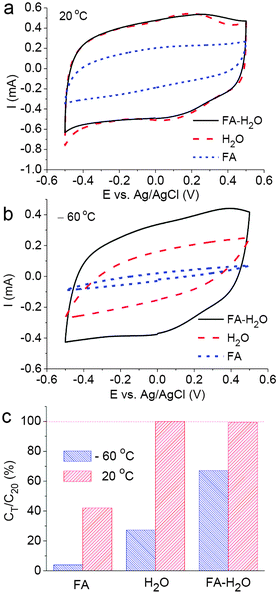
Fig. 1 compares the CVs and specific capacitances of the CNT electrode measured in different electrolytes at 20 °C and −60 °C. Fig. 1a shows that at 20 °C the CNT electrode behaved almost identically in the CaCl2–H2O and CaCl2–FA–H2O solutions with the CV being highly rectangular in shape, suggesting an insignificant effect of FA on the capacitance of CNTs. In the CaCl2–FA solution, the CV currents were much smaller, which could be due to the applied CaCl2 concentration (1 mol L−1) was already close to saturation and the solution was very viscous. Decreasing the temperature caused a significant impact on the CVs recorded in the CaCl2–H2O and CaCl2–FA solutions, but much less so in CaCl2–FA–H2O as exemplified in Fig. 1b. Surprisingly, CVs were still recorded in CaCl2–H2O at temperatures below the reported eutectic point, −51.6 °C. These highly reproducible CVs did show noticeably increased resistance in the cell (indicated by the sluggish current switching at both ends of the potential scan), but the currents were not insignificant. This observation indicates the presence of liquid in the cell, likely due to supercooling of the CaCl2–H2O solution (see ESI†).
The capacitance retention (CT/C20 = 100 × capacitance at designated temperature/capacitance at 20 °C) of the CNT electrode as derived from the rectangular CVs (see ESI†) is presented in Fig. 1c. At 20 °C, the measured specific capacitance, Csp, was almost the same in CaCl2–H2O (108.3 F g−1) and CaCl2–FA–H2O (107.6 F g−1). In this work, it was observed that the acid treated CNTs exhibited 30–50% higher capacitance in the aqueous solution of CaCl2 than that of KCl (see Fig. S4 in the ESI†). This difference can be attributed to the greater affinity of the Ca2+ ions towards the oxy-groups on the CNT surfaces as mentioned above. The fact that each Ca2+ ion has two positive charges could be another important cause for the increase of the specific capacitance. More discussion is given later and also in the ESI.†
The dependence of specific capacitance on temperature was studied next. In the temperature range from 20 to −70 °C, only the CaCl2–FA–H2O solution could remain as a liquid, which is needed to ensure ion mobility and accessibility to the electrode surfaces. Consequently, a meaningful specific capacitance was achieved (72.7 F g−1 or 67% capacitance retention at −60 °C, see Fig. 1c). Fig. 2a shows the CVs of the CNT electrode in the CaCl2–FA–H2O electrolyte at temperatures varying from 20 to −70 °C. In the experiment, the potential was cycled continuously, and the CV was recorded at each of the indicated temperatures in Fig. 2a. It can be seen that the CNT electrode exhibited highly satisfactory capacitive behaviour (rectangular shape) in the applied temperature range. The CVs decreased in current as temperature decreased, corresponding to the variation of specific capacitance as shown in Fig. 2b. It can be noted that at −70 °C, the CV is notably separated from that recorded at −60 °C, which can be explained by the CaCl2–FA–H2O solution under supercooling (see Fig. S1 and S2 in ESI†). Even though, the specific capacitance of the CNT electrode was still measured to be as high as 53.4 F g−1 or 51% capacitance retention. The cause for the capacitance decay with decreasing temperature may be related to the reduced mobility of ions at lower temperatures. A further contribution to lowering the ion mobility may come from the organoaqueous solution becoming more viscous upon cooling and supercooling.
 | ||
Fig. 2 (a) CVs and (b) CT/C20 of a CNT electrode in the 2.0 mol L−1 solution of CaCl2 in mixed FA–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v 1 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) at different temperatures as indicated. Potential scan rate: 100 mV s−1. v) at different temperatures as indicated. Potential scan rate: 100 mV s−1. | ||
Obviously, the dry ice container is not able to offer equilibrium conditions at temperatures higher than that of dry ice. However, as stated above, once placed in the dry ice container, particularly upon the effect of opening and closing the container lid, the electrolyte temperature dropped fairly slowly. At a rate smaller than 1 °C min−1, the difference between CVs continuously recorded at 100 mV s−1 was very much insignificant. Therefore, the results presented in Fig. 2 can be regarded as recorded under a pseudo-steady state, serving as a practically useful guideline.
The higher specific capacitance of CNTs measured in aqueous CaCl2 electrolytes than in KCl warrants more discussion. According to theory, electric double layer capacitance should increase with electrolyte concentration.9a This trend agrees with some previous studies of activated carbon in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type electrolyte such as KCl and KOH in which a double capacitance increase required a 10 fold or greater increase of electrolyte concentration.9b,c To compare a 1
1 type electrolyte such as KCl and KOH in which a double capacitance increase required a 10 fold or greater increase of electrolyte concentration.9b,c To compare a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type electrolyte with a 1
1 type electrolyte with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 type, e.g. KCl vs. CaCl2, a simple way was adopted in this work to half the concentration of the latter, e.g. 1.0 mol L−1 KCl vs. 0.5 mol L−1 CaCl2. The results obtained are presented in Fig. S4 in the ESI,† showing clearly higher currents on the CVs recorded in the CaCl2 solution at both concentrations. Note that the CNT electrode responded much more significantly to replacing KCl with CaCl2 than to doubling the electrolyte concentration. Thus, as discussed above and further in the ESI† (Fig. S6), we believe that the unique interactions between Ca2+ ions and the CNT surface oxy-groups could have played important roles. This understanding is new to supercapacitor research and, if further proven, could have a significant impact on the development of supercapacitors based on CNTs, graphenes and/or activated carbons which are all enriched with various surface oxy-groups.
2 type, e.g. KCl vs. CaCl2, a simple way was adopted in this work to half the concentration of the latter, e.g. 1.0 mol L−1 KCl vs. 0.5 mol L−1 CaCl2. The results obtained are presented in Fig. S4 in the ESI,† showing clearly higher currents on the CVs recorded in the CaCl2 solution at both concentrations. Note that the CNT electrode responded much more significantly to replacing KCl with CaCl2 than to doubling the electrolyte concentration. Thus, as discussed above and further in the ESI† (Fig. S6), we believe that the unique interactions between Ca2+ ions and the CNT surface oxy-groups could have played important roles. This understanding is new to supercapacitor research and, if further proven, could have a significant impact on the development of supercapacitors based on CNTs, graphenes and/or activated carbons which are all enriched with various surface oxy-groups.
In summary, we have demonstrated that mixing CaCl2, FA and H2O can enable not only very low freezing temperatures, but also excellent capacitive charge storage in CNTs at both room and sub-zero temperatures below −60 °C. The specific capacitance of the CNTs measured in CaCl2 based electrolytes is higher than that in more commonly used alkali salt based electrolytes. This improvement can be largely attributed to the unique affinity between calcium ions and the oxy-groups on the surfaces of carbon nanotubes. We hope that this report can encourage further research on using CaCl2 based electrolytes to improve the performance of other carbon materials in supercapacitors.
This work has received financial support from the China Scholarship Council, the EPSRC (EP/J000582/1), and the Ningbo Municipal Government (3315 Plan and the IAMET Special Fund, 2014A35001-1).
Notes and references
- (a) R. Kotz and M. Carlen, Electrochim. Acta, 2000, 45, 2483–2498 CrossRef CAS; (b) E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC; (c) P. Simon and Y. Gogotsi, Acc. Chem. Res., 2013, 46, 1094–1103 CrossRef CAS PubMed; (d) G. Z. Chen, Prog. Nat. Sci.-Mater. Int., 2013, 23, 245–255 CrossRef PubMed.
- (a) S. J. Guo and S. J. Dong, Chem. Soc. Rev., 2011, 40, 2644–2672 RSC; (b) G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC; (c) W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- (a) P. Ruch, D. Cericola, A. Foelske, R. Kötz and A. Wokaun, Electrochim. Acta, 2010, 55, 2352–2357 CrossRef CAS PubMed; (b) D. Weingarth, H. Noh, A. Foelske-Schmitz, A. Wokaun and R. Kötz, Electrochim. Acta, 2013, 103, 119–124 CrossRef CAS PubMed; (c) M. Lazzari, F. Soavi and M. Mastragostino, Fuel Cells, 2010, 10, 840–847 CrossRef CAS PubMed.
- (a) J. Xu, R. Zhang, P. Chen and S. Ge, J. Power Sources, 2014, 246, 132–140 CrossRef CAS PubMed; (b) S. Vijayakumar, S. Nagamuthu and G. Muralidharan, ACS Appl. Mater. Interfaces, 2013, 5, 2188–2196 CrossRef CAS PubMed; (c) B. Mendoza-Sánchez, T. Brousse, C. Ramirez-Castro, V. Nicolosi and P. S. Grant, Electrochim. Acta, 2013, 91, 253–260 CrossRef PubMed.
- (a) E. Perricone, M. Chamas, L. Cointeaux, J.-C. Leprêtre, P. Judeinstein, P. Azais, F. Béguin and F. Alloin, Electrochim. Acta, 2013, 93, 1–7 CrossRef CAS PubMed; (b) W.-Y. Tsai, R. Lin, S. Murali, L. L. Zhang, J. K. McDonough, R. S. Ruoff, P.-L. Taberna, Y. Gogotsi and P. Simon, Nano Energy, 2013, 2, 403–411 CrossRef CAS PubMed; (c) E. Iwama, P.-L. Taberna, P. Azais, L. Brégeon and P. Simon, J. Power Sources, 2012, 219, 235–239 CrossRef CAS PubMed.
- (a) A. J. Roberts, A. F. D. Namor and R. C. T. Slade, Phys. Chem. Chem. Phys., 2013, 15, 3518–3526 RSC; (b) Y.-T. Weng, H.-A. Pan, N.-L. Wu and G. Z. Chen, J. Power Sources, 2015, 274, 1118–1125 CrossRef CAS PubMed; (c) TATA Chemicals Europe, Calcium Chloride – Winter De-Icing, http://www.tatachemicals.com/europe/products/pdf/Calcium_Chloride/information_deicing.pdf, viewed on 09 March 2015.
- (a) A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011 RSC; (b) E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed; (c) Q. Zhang, K. O. Viqier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- (a) G. Z. Chen, M. S. P. Shaffer, D. Coleby, G. Dixon, W. Zhou, D. J. Fray and A. H. Windle, Adv. Mater., 2000, 12, 522–526 CrossRef CAS; (b) C. Peng, G. A. Snook, D. J. Fray, M. S. P. Shaffer and G. Z. Chen, Chem. Commun., 2006, 4629–4631 RSC; (c) C. Peng, J. Jin and G. Z. Chen, Electrochim. Acta, 2007, 53, 525–537 CrossRef CAS PubMed.
- (a) A. J. Bard and L. R. Faulkner, Electrochemical methods: Fundamentals and applications, John Wiley & Sons, Inc., New York, 2nd edn, 2001 Search PubMed; (b) Y. Tian, J.-W. Yan, R. Xue and B.-L. Yi, Acta Phys.-Chem. Sin., 2011, 27, 479–485 CAS; (c) J. H. Chae and G. Z. Chen, Particuology, 2013, 15, 9–17 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc03048j |
| This journal is © The Royal Society of Chemistry 2015 |