Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Titanium migration driven by Li vacancies in Li1−xTi2O4 spinel†
A.
Kitada
a,
A. M.
Arevalo-Lopez
b and
J. P.
Attfield
*b
aDepartment of Materials Science and Engineering, Kyoto University, Yoshida-honmachi, Sakyo, Kyoto 606-8501, Japan
bCentre for Science at Extreme Conditions (CSEC) and School of Chemistry, University of Edinburgh, Edinburgh EH9 3FD, UK. E-mail: j.p.attfield@ed.ac.uk
First published on 28th May 2015
Abstract
Gentle oxidation of lithium titanate spinel (LiTi2O4) with water at room temperature gives Li-deficient Li0.33Ti2O4. Combined X-ray and neutron Rietveld analysis shows that 28% of the Ti cations are displaced to alternative octahedral sites, in keeping with a proposed model based on Ti-migration limited by Li-vacancy concentration.
Lithium transition metal oxide spinels have important physical and electrochemical properties. LiTi2O4 spinel has been extensively studied as it displays superconductivity with a critical temperature of 11 K.1–6 Lithium titanate spinels also have attractive properties as negative electrodes for lithium ion batteries such as a very flat potential and a relatively low volume change upon charging–discharging and with a good rate performance.7–13
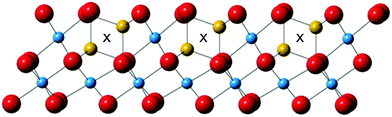
The cubic spinel structure of LiTi2O4 consists of cubic-close-packed (ccp) oxide layers with Li cations occupying tetrahedral sites (with symmetry label 8a in the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group) and Ti cations in 16d octahedral sites, and is written here as (Li)8a[Ti2]16dO4. Alternative 16c octahedral cation sites (marked ‘X’ on Fig. 1) close to the 8a sites are empty and define a three-dimensional network of channels through which lithium ions diffuse during electrochemical reactions. Intercalation of additional lithium into LiTi2O4 results in migration of Li cations from 8a to 16c sites, giving an ordered rocksalt type product denoted as {Li2}16c[Ti2]16dO4.5,8,14
m space group) and Ti cations in 16d octahedral sites, and is written here as (Li)8a[Ti2]16dO4. Alternative 16c octahedral cation sites (marked ‘X’ on Fig. 1) close to the 8a sites are empty and define a three-dimensional network of channels through which lithium ions diffuse during electrochemical reactions. Intercalation of additional lithium into LiTi2O4 results in migration of Li cations from 8a to 16c sites, giving an ordered rocksalt type product denoted as {Li2}16c[Ti2]16dO4.5,8,14
The structural consequences of delithiating LiTi2O4 to form Li1−xTi2O4 are less well understood. Electrochemical delithiation was found to be limited to a deficiency of x ∼ 0.3 due to oxidative decomposition of the electrolyte3 so chemical methods have mainly been used. An early study showed delithiation up to x = 0.8 could be achieved with iodine in acetonitrile. The product Li0.2Ti2O4 was reported to be a cation-disordered defect rocksalt material (cubic, a = 4.116 Å) without spinel superstructure peaks, indicating that 16c and 16d sites have equal cation occupancies.8 A wide two-phase region between x ≈ 0.15 and x ≈ 0.7 has subsequently been reported.6 Samples in the small-x region maintain the spinel cation arrangement although a small amount of Ti migration was observed, e.g. 1.8% Ti migration from 16d to 16c sites at x = 0.14.4 The x ≈ 0.7 product was reported to have substantial Ti occupancy of the 16c octahedral sites and also the tetrahedral 8a sites.6 However both oxidation methods used gave secondary phase impurities; oxidation by air introduced an amorphous carbonate impurity and Br2 or I2 treatments led to a non-spinel Li1−yTi2−yO3 secondary phase. A simple oxidation method to generate high purity material is thus needed to determine the structure and degree of cation migration in Li1−xTi2O4 accurately.
Here we report the preparation of lithium-deficient disordered spinel Li0.33Ti2O4 by mild oxidation through immersion in water at room temperature (RT). The sample has been characterized by chemical and thermogravimetric (TGA) analysis, and combined powder X-ray and neutron structure refinement has been used to establish the cation distribution from which a likely migration mechanism is proposed.
The LiTi2O4 spinel precursor was prepared by a reported solid state method.2 Immersion of LiTi2O4 in water for short periods of a few days was found to produce two-phase spinel samples like those reported previously.6 To obtain the x ≈ 0.7 phase, 0.1608 g of LiTi2O4 precursor was delithiated chemically by reaction with distilled water (100.0 ml) in a capped flask for 2 weeks at RT. Bubbles were seen when the precursor was immersed due to evolution of H2 and the solution became strongly basic (pH = 12). The reaction is;
| LiTi2O4 + xH2O → Li1−xTi2O4 + (x/2)H2 + xLiOH, |
Powder X-ray diffraction (PXRD) data from a Bruker D2 instrument using CuKα radiation displayed in Fig. 2 show only spinel peaks from the Li0.33Ti2O4 sample with no secondary phases, unlike products in ref. 6. Substantial diffuse scattering in the background evidences disorder of the heavy Ti atoms within the spinel lattice. Rietveld fits showed that Ti is disordered over octahedral 16c and 16d sites, but did not give a significant improvement (Rwp decreased slightly to 5.71 from 5.76%) when Ti was also allowed to occupy the tetrahedral 8a Li sites as proposed elsewhere,6 so this possibility was not considered further.
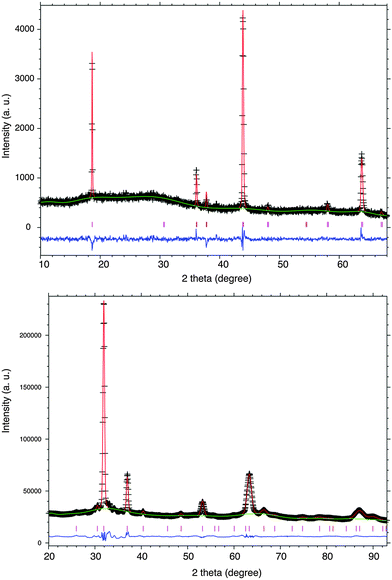 | ||
| Fig. 2 Rietveld fits from the combined refinement of Li0.33Ti2O4 against PXRD (upper, weighted profile residual Rwp = 5.69%) and PND (lower, Rwp = 2.06%) profiles at RT. | ||
Powder neutron diffraction (PND) data were collected from 0.1 g of the Li0.33Ti2O4 sample on instrument D20 at ILL Grenoble with a neutron wavelength of 1.3029(4) Å. Combined fits to the PXRD and PND profiles using the GSAS package15 were used to test further Li/Ti disorder models. The total lithium content was fixed to 0.33 in keeping with the ICP-AES and TGA results. Ti was found to be disordered over 16c and 16 d sites. Models where Li was allowed to occupy alternative 8b tetrahedral sites, or co-occupy octahedral 16c or 16d positions with Ti gave insignificant or negative Li occupancies at those sites. Hence we conclude that the average structure of the Li-deficient Li1−xTi2O4 spinel phase is best described by the model shown in Table 1, with Li at tetrahedral 8a sites only, and Ti at octahedral 16c and 16d sites with respective 28 and 72% occupancies for Li0.33Ti2O4.† The Li–O distance is 1.867(2) Å and Ti(16c)–O and Ti(16d)–O distances are 2.113(2) and 2.019(1) Å. The predicted Ti3.83+–O length of 1.983 Å based on ionic radii16 is close to the Ti(16d)–O value, but the Ti(16c)–O distance is long and suggests that local rearrangement occurs around occupied 16c sites.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (No. 227). The cubic cell parameter is a = 8.259(3) Å
m (No. 227). The cubic cell parameter is a = 8.259(3) Å
| Atom | Site | x | y | z | Occupancy | U iso (Å2) |
|---|---|---|---|---|---|---|
| Li(1) | 8a | 0.125 | 0.125 | 0.125 | 0.33 | 0.0442(8) |
| Ti(1) | 16d | 0.5 | 0.5 | 0.5 | 0.719(8) | 0.0442 |
| Ti(2) | 16c | 0 | 0 | 0 | 0.281 | 0.0442 |
| O(1) | 32e | 0.2557(2) | 0.2557 | 0.2557 | 1.00 | 0.0273(4) |
Substantial migration of Ti cations during a reaction at RT is surprising in view of the strength of Ti–O bonds, but this effect is consistently observed in Li1−xTi2O4 spinels, and a similar disorder was reported in delithiated LiV2O4 spinel with cation distribution (Li0.28V0.1)8a{V0.4}16c[V1.5]16dO4.17 Migration of V to tetrahedral 8a sites was attributed to V5+ formation, but tetrahedral coordination of Ti4+ is less likely and is not evidenced in our study for Li0.33Ti2O4. It is evident that the hypothetical fully delithiated [Ti2]16dO4 spinel form of TiO2 is highly unstable. This has been explained through first principles calculations which confirm that the hypothetical Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m [Ti2]16dO4 polymorph is much less stable than rutile, anatase, brookite, ramsdellite, or the high pressure α-PbO2 forms of TiO2.18 In the case of manganese, by contrast, [Mn2]16dO4 with Fd
m [Ti2]16dO4 polymorph is much less stable than rutile, anatase, brookite, ramsdellite, or the high pressure α-PbO2 forms of TiO2.18 In the case of manganese, by contrast, [Mn2]16dO4 with Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry has been proven to be stable both experimentally and theoretically.19–21 This shows that Ti cation size as well as charge is significant in destabilising [Ti2]16dO4.
m symmetry has been proven to be stable both experimentally and theoretically.19–21 This shows that Ti cation size as well as charge is significant in destabilising [Ti2]16dO4.
We note that a straightforward explanation for the migration of octahedral cations in delithiated Li1−xTi2O4 spinels can be given on the basis of the occupancy of octahedral sites between close-packed oxide layers. Several kinds of stacking are observed among stable TiO2 polymorphs; rutile, ramsdellite and the α-PbO2 form are close to hexagonal close-packing (hcp), anatase has ccp, and brookite has a mixed ccp–hcp sequence.5 Ti4+ ions occupy half of the octahedral sites between each pair of oxide layers in all of these structures. This provides a key distinction from the hypothetical [Ti2]16dO4 spinel polymorph. The LiTi2O4 spinel structure has two alternating types of layer in terms of cation occupancy as shown in Fig. 1. One type (labelled Layer 1) has 3/4 of the octahedral sites filled by Ti, while the other (Layer 2) has 1/4-filling of octahedral Ti sites plus half the tetrahedral sites occupied by Li. Complete delithiation to give [Ti2]16dO4 would leave a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Layer 1
1 Layer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Layer 2 ratio of occupied octahedral sites which is highly disfavoured due to cation–cation repulsions with respect to 1
Layer 2 ratio of occupied octahedral sites which is highly disfavoured due to cation–cation repulsions with respect to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 distributions. The alternative 16c sites in spinel have a 1
1 distributions. The alternative 16c sites in spinel have a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 Layer 1
3 Layer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Layer 2 ratio, so migration from 16d to 16c sites is a mechanism for equalizing the filling of octahedral sites in the two layer types. The observed 16d and 16c Ti occupancies of 72 and 28% in our refinement of Li0.33Ti2O4 are equivalent to a Layer 1
Layer 2 ratio, so migration from 16d to 16c sites is a mechanism for equalizing the filling of octahedral sites in the two layer types. The observed 16d and 16c Ti occupancies of 72 and 28% in our refinement of Li0.33Ti2O4 are equivalent to a Layer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Layer 2 octahedral Ti ratio of 0.61
Layer 2 octahedral Ti ratio of 0.61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.39, showing that the populations are approaching equality.
0.39, showing that the populations are approaching equality.
To consider if there is any quantitative relationship between Li deficiency and the degree of Ti migration in Li1−xTi2O4, we write the cation distribution as (Li1−x)8a{Tiy}16c[Ti2−y]16dO4. The boundary conditions are y = 0 at x = 0, as no migration is observed in stoichiometric LiTi2O4, and y = 1 at x = 1 as cation–cation repulsions favour equal Layer 1 and Layer 2 populations as noted above (corresponding to a hypothetical {Ti}16c[Ti]16dO4 disordered and defective rocksalt polymorph of TiO2). The simplest relationship y = x is in poor agreement with the observed y = 0.56 (twice the 16c occupancy) for x = 0.67 for our Li0.33Ti2O4 sample. A better description requires consideration of interactions between Li and Ti cations where clustering leads to y < x. Each 16c site is only 1.79 Å from two 8a Li sites, so both of these tetrahedral sites have to be vacant for the 16c site to be occupied by Ti (see Fig. 1). Hence we propose that for small x, local defect clusters of two Li vacancies surrounding an interstitial Ti at 16c are formed. This assumption gives y = x/2 at small x. Combining this with the above boundary conditions gives a simple quadratic variation y = (x/2)(1 + x), which provides an approximate description of greater degrees of clustering at high x. For our highly deficient x = 0.67 sample this equation predicts y = 0.56 (16c Ti occupancy = 0.28), in perfect agreement with the observed populations in Table 1. This indicates that Ti migration from 16d to 16c sites in highly Li-deficient Li1−xTi2O4 is limited by the distribution of remaining Li cations, with Li vacancies clustering around Ti cations on 16c sites.
For Li0.86Ti2O4 which represents the distinct small Li-deficiency (x < 0.15) phase, the y = 0.08 value predicted by the clustering model is somewhat larger than the reported value of 0.04.4 This suggests that defect clustering may drive the phase separation observed between x ≈ 0.15 and x ≈ 0.7 in the Li1−xTi2O4 system,6 with very little Ti migration in the small-x region, and a cascade effect above the x ≈ 0.15 limit where Li vacancies and 16c Ti cations cluster in small areas with large x ≈ 0.7.
Clustering of vacancies and cations is reported in many other solids and may be associated with electronic effects such as charge order or metal–metal bonding. For example, in Na1−xCoO2, redistribution of Na+ into two prismatic sites between CoO2 layers is accompanied by Co3+/Co4+ charge ordering,22 while in KNi2Se2 Ni–Ni bonding is an electronic driving force for vacancy formation and Ni migration into adjacent K layers.23 Hence Ti–Ti bonding8 and local Ti3+/Ti4+ charge order may also play a part in the Ti migration in LiTi2O4.
In conclusion, water oxidation is demonstrated to be a useful method for obtaining Li-deficient Li1−xTi2O4 spinels without decomposition to other Ti oxide phases. Combined PXRD and PND refinement shows that Li0.33Ti2O4 has Li at tetrahedral sites only, but Ti is disordered over two octahedral positions. This Ti migration acts as a mechanism for equalizing the filling of octahedral sites between close-packed oxide layers to minimize cation–cation repulsions. The wide immiscibility gap between x ≈ 0.15 and 0.7 is driven by clustering of Li vacancies and 16c Ti cations. Further microstructural and computational investigations of defect clustering and phase segregation will be useful to add further insights.
We thank EPSRC, STFC and the Kyoto University Global Frontier Project for Young Professionals (John-Mung Advanced Program) for support, and C. Ritter for assistance with data collection at ILL.
Notes and references
- D. C. Johnston, H. Prakash, W. H. Zachariasen and R. Viswanathan, Mater. Res. Bull., 1973, 8, 777 CrossRef CAS.
- Y. Ueda, T. Tanaka, K. Kosuge, M. Ishikawa and H. Yasuoka, J. Solid State Chem., 1988, 77, 401 CrossRef CAS.
- S. Hamada, M. Kato, T. Noji and Y. Koike, Physica C, 2010, 470, 5766 CrossRef PubMed.
- E. G. Moshopoulou, J. Am. Ceram. Soc., 1999, 82, 3317 CrossRef CAS PubMed.
- D. W. Murphy, R. J. Cava, S. M. Zahurak and A. Santoro, Solid State Ionics, 1983, 9–10, 413 CrossRef CAS.
- M. Rygula, S. Kemmler-Sack, T. Nissel and R. P. Hübener, Ann. Phys., 1993, 505, 685 CrossRef PubMed.
- K. M. Colbow, J. R. Dahn and R. R. Haering, J. Power Sources, 1989, 26, 397 CrossRef CAS.
- R. J. Cava, D. W. Murphy, S. Zahurak, A. Santoro and R. S. Roth, J. Solid State Chem., 1984, 53, 64 CrossRef CAS.
- M. Manickam and M. Takata, J. Power Sources, 2003, 114, 298 CrossRef CAS.
- X. Sun, P. V. Radovanovic and B. Cui, New J. Chem., 2015, 39, 38 RSC.
- J. Qiu, C. Lai, E. Gray, S. Li, S. Qiu, E. Strounina, C. Sun, H. Zhao and S. Zhang, J. Mater. Chem. A, 2014, 2, 6353 CAS.
- S. Chen, Y. Xin, Y. Zhou, Y. Ma, H. Zhou and L. Qi, Energy Environ. Sci., 2014, 7, 1924 CAS.
- J. Guo, W. Zuo, Y. Cai, S. Chen, S. Zhang and J. Liu, J. Mater. Chem. A, 2015, 3, 4938 CAS.
- J. Yang, J. Zhao, Y. Chen and Y. Li, Ionics, 2010, 16, 425 CrossRef CAS.
- A. C. Larson, R. B. Von Dreele, General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR 86-748, 2000.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- L. A. de Picciotto and M. M. Thackeray, J. Power Sources, 1991, 35, 323 CrossRef.
- W. C. Mackrodt, J. Solid State Chem., 1999, 142, 428 CrossRef CAS.
- J. C. Hunter, J. Solid State Chem., 1981, 39, 142 CrossRef CAS.
- J. E. Greedan, N. P. Raju, A. S. Wills, C. Morin and S. M. Shaw, Chem. Mater., 1998, 10, 3058 CrossRef CAS.
- S. K. Mishra and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 6120 CrossRef CAS.
- Y. S. Meng, A. Van der Ven, M. K. Y. Chan and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 172103 CrossRef.
- J. R. Neilson and T. M. McQueen, J. Am. Chem. Soc., 2012, 134, 750 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Cif file for the combined PXRD and PND refinement of Li0.33Ti2O4. See DOI: 10.1039/c5cc02800k |
| This journal is © The Royal Society of Chemistry 2015 |