Open Access Article
Open Access ArticleEnergy migration within hexameric hemoprotein reconstituted with Zn porphyrinoid molecules†
Koji
Oohora
a,
Tsuyoshi
Mashima
a,
Kei
Ohkubo
bc,
Shunichi
Fukuzumi
bcd and
Takashi
Hayashi
*a
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita 565-0871, Japan. E-mail: thayashi@chem.eng.osaka-u.ac.jp
bDepartment of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA and SENTAN, Japan Science and Technology (JST), Suita, Osaka 565-0871, Japan
cDepartment of Bioinspired Science, Ewha Womans University, Seoul 120-750, Korea
dFaculty of Science and Technology, Meijo University, ALCA and SENTAN, Japan Science and Technology (JST), Nagoya, Aichi 468-8502, Japan
First published on 2nd June 2015
Abstract
Photosensitizers, Zn protoporphyrin IX and Zn chlorin e6, are completely inserted into each heme pocket of a hexameric apohemoprotein. The fluorescence quenching efficiencies upon addition of methyl viologen are 2.3 and 2.6 fold-higher than those of the partially photosensitizer-inserted proteins, respectively, indicating that the energy migration occurs within the proteins.
An array of natural pigments achieves efficient capture of sunlight in natural photosynthetic systems.1 For example, LH2, a simple light harvesting complex from purple bacterium containing a precise array of eighteen Mg-bacteriochlorin molecules, demonstrates energy migration via successive and rapid energy transfer within protein matrices.2 To mimic such a structure and function, a number of efforts to reproduce an array of photosensitizers (especially porphyrin derivatives) have been undertaken using synthetic,3 supramolecular,4 and coordination-bonding5 approaches. Several proteins have also been found to provide appropriate scaffolds for accumulating photosensitizers by supramolecular interactions6 or covalent modifications.7 In this context, we have focused on the use of the native oligomer of hexameric tyrosine-coordinated heme protein (HTHP) from the marine bacterium Silicibacter pomeroyi8 as a scaffold for construction of a new array of photosensitizers. Dobbek and coworkers reported that HTHP is a C6-symmetric toroidal hexamer with each domain including a 75-residue peptide chain consisting of three α-helices and a non-covalently bound iron protoporphyrin IX, heme b cofactor. According to the crystal structure, the heme–heme distance is 18 Å (Fig. 1b). Although the native function of HTHP remains unknown, the unique structure and its peroxidase and catalase activities have been investigated.8 Here, we report that reconstitution of the hemoprotein with zinc porphyrinoid complexes9 provides an array of photosensitizers with well-defined orientations in the HTHP matrices (Fig. 1). The modified HTHP is found to provide a model of the light harvesting complex which demonstrates energy migration within an array of chromophores. The present system will serve as the versatile strategy to create a light harvesting complex based on a series of porphyrinoid photosensitizers.
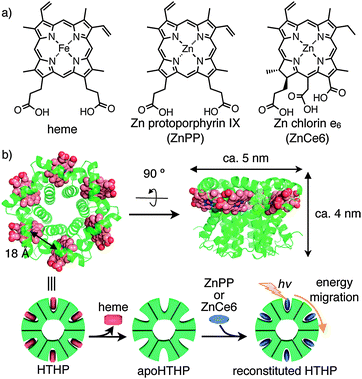 | ||
| Fig. 1 (a) Molecular structures of heme and Zn porphyrinoids, ZnPP and ZnCe6. (b) Crystal structure of HTHP (PDB ID; 2OYY) and schematic representation of the preparation of reconstituted HTHP. | ||
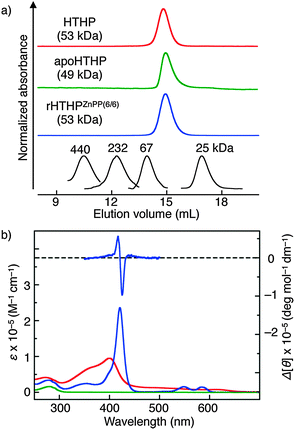
HTHP is expressed in a recombinant E. coli system and purified by anion exchange and size exclusion chromatography (SEC). Analytical SEC (Fig. 2a) and DLS (dynamic light scattering, Table S1, ESI†) reveal a monodisperse species with a hydrodynamic diameter of 5.4 nm, which is consistent with the value expected from the hexameric structure observed in X-ray crystallography,8 and ESI-TOF MS (Fig. S1, ESI†) shows the desired mass numbers of the multiply ionized holo-hexameric species: found m/z = 3318.2 and 3539.4; calcd m/z = 3318.5 (z = 16+) and 3539.7 (z = 15+). The apo-form of HTHP (apoHTHP) was prepared by a conventional method using acid and 2-butanone,10 and the resulting protein has no absorption in the visible region (Fig. 2b). The hexameric structure in the apo-form was confirmed by analytical SEC and DLS measurements. In addition, the CD spectrum of apoHTHP in the far-UV region is consistent with that of HTHP, showing that the α-helices are maintained in the absence of the heme cofactors (Fig. S2, ESI†). Addition of excess amounts of Zn protoporphyrin IX (ZnPP) into an apoHTHP solution under pH-neutral conditions yields reconstituted HTHP (rHTHPZnPP(6/6)), where 6/6 represents the complete incorporation of the zinc complex into the six heme pockets in apoHTHP.11,12 The UV-vis absorption spectrum of rHTHPZnPP(6/6) has maxima at 421, 548, and 584 nm (Fig. 2b). This pattern is similar to that of tyrosine-coordinated ZnPP in human serum albumin.13 Analytical SEC measurements for rHTHPZnPP(6/6) indicate that the protein has the same elution volume as HTHP. DLS measurements indicate a hydrodynamic diameter of 5.6 nm and confirm that the thermodynamically stable hexameric structure is maintained.
Addition of apoHTHP into a rHTHPZnPP(6/6) aqueous solution increases the intensity of fluorescence derived from ZnPP moieties (Fig. S4, ESI†), indicating re-equilibration toward reconstituted HTHP with less than six ZnPP molecules, rHTHPZnPP(n/6), where n represents the apparent number of the photosensitizer molecules in the six heme pockets.14 The fluorescence lifetime (τ) of rHTHPZnPP(6/6) was determined to be 1.43 ± 0.01 ns, which is slightly shorter than that of rHTHPZnPP(1/6) (τ = 1.56 ± 0.01 ns). Taken together with lower fluorescence intensity in rHTHPZnPP(6/6), it appears that singlet–singlet annihilation occurs in the protein hexamer.3a–c,4c,15 The visible absorption spectrum of rHTHPZnPP(n/6) is similar to that of rHTHPZnPP(6/6), indicating that the coordination environments of the two proteins are similar (Fig. S5, ESI†). This re-equilibration is also confirmed by a differential CD spectrum obtained by subtracting the spectrum of rHTHPZnPP(1/6) from that of rHTHPZnPP(6/6). The observed split type Cotton effect (Fig. 2b) induced by ZnPP–ZnPP exciton coupling strongly suggests the formation of conformationally-defined Zn porphyrin arrays.16–18 These findings also indicate that the ZnPP molecules can be incorporated into each subunit of apoHTHP while maintaining the intrinsic hexameric structure, whereas re-equilibration upon addition of apoHTHP provides a mixture of incompletely-reconstituted photosensitizer-containing proteins.
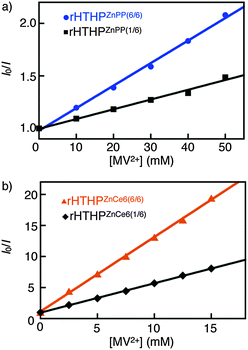
Stern–Volmer plots of steady-state and time-resolved emission against the concentration of methyl viologen dichloride (MV2+) are shown in Fig. 3a and Fig. S8, ESI,† respectively. Quenching of steady state fluorescence by MV2+ (Fig. S9, ESI†) was observed at relatively high concentrations ([MV2+] >1 mM), whereas no changes in lifetimes upon the addition of MV2+ were observed (Fig. S10, ESI†).19 This indicates static quenching of fluorescence of rHTHPZnPP(n/6) by MV2+. The slopes of the Stern–Volmer plots for steady state fluorescence of rHTHPZnPP(n/6) were determined to be 21 M−1 (n = 6) and 9.2 M−1 (n = 1) as apparent binding constants. The actual binding constant of MV2+ for rHTHPZnPP(6/6) evaluated by UV-vis spectral changes to form the charge-transfer complex (Fig. S11, ESI†) is consistent with that of rHTHPZnPP(1/6) (ca. 9 M−1). Taken together, these results indicate that the higher apparent value of the protein hexamer fully occupied by photosensitizers is a result of efficient quenching which occurs due to the energy migration within the ZnPP molecule array.20
Similar results were obtained using Zn chlorin e6 (ZnCe6) instead of ZnPP (Fig. 1a). The reconstituted protein, rHTHPZnCe6(6/6), was also characterized by analytical SEC, DLS, UV-vis and CD spectroscopic measurements (Fig. S13 and Table S1, ESI†). The fluorescence intensity of rHTHPZnCe6(n/6) is found to depend on the n value, the ratio of the bound photosensitizer for apoHTHP. The apparent binding constants of MV2+ derived from the Stern–Volmer plots for steady state fluorescence of rHTHPZnCe6(6/6) and rHTHPZnCe6(1/6) are 1.2 × 103 M−1 and 4.7 × 102 M−1, respectively (Fig. 3b). In contrast, the actual binding constants of MV2+ for rHTHPZnCe6(n/6) determined by UV-vis spectral changes (Fig. S15, ESI†) are 5 × 102 M−1 (n = 6) and 4 × 102 M−1 (n = 1).21 Therefore, the 2.6-fold greater apparent binding constant of rHTHPZnCe6(6/6) relative to rHTHPZnCe6(1/6) suggests that the energy migration occurs within the ZnCe6 array as well as rHTHPZnPP(6/6).20
In conclusion, the present study demonstrates that the oligomeric hemoprotein is a versatile and useful model for detecting energy migration within assembled porphyrinoid photosensitizers with well-defined orientations in the heme binding sites. The present system is expected to contribute to generation of new efficient photo-catalysts and devices which harness the biological light harvesting function.
We gratefully acknowledge Prof. M. J. Crossley (Univ. Sydney) for helpful discussion. This work was supported by the Sekisui Chemical Grant Program from Research (Japan), the Asahi Glass Foundation (Japan) and Grants-in-Aid (25810099, 26104523, 24655051, 22105013, 26620154, 26288037 and 15H00873) provided by JSPS and MEXT. This research was also supported by SICORP (JST). T.M. appreciates support from the JSPS Research Fellowship for Young Scientists.
Notes and references
- G. D. Scholes, G. R. Fleming, A. Olaya-Castro and R. van Grondelle, Nat. Chem., 2011, 3, 763–774 CrossRef CAS PubMed.
- G. McDermott, S. M. Prince, A. A. Freer, A. M. Hawthornthwaite-Lawless, M. Z. Papiz, R. J. Cogdell and N. W. Isaacs, Nature, 1995, 374, 517–521 CrossRef CAS.
- (a) F. Hajjaj, Z. S. Yoon, M.-C. Yoon, J. Park, A. Satake, D. Kim and Y. Kobuke, J. Am. Chem. Soc., 2006, 128, 4612–4623 CrossRef CAS PubMed; (b) N. Aratani, D. Kim and A. Osuka, Acc. Chem. Res., 2009, 42, 1922–1934 CrossRef CAS PubMed; (c) J. Yang, M.-C. Yoon, H. Yoo, P. Kim and D. Kim, Chem. Soc. Rev., 2012, 41, 4808–4826 RSC; (d) S. Fukuzumi and K. Ohkubo, J. Mater. Chem., 2012, 22, 4575–4587 RSC; (e) S. Cho, W.-S. Li, M.-C. Yoon, T. K. Ahn, D.-L. Jiang, J. Kim, T. Aida and D. Kim, Chem. – Eur. J., 2006, 12, 7576–7584 CrossRef CAS PubMed; (f) S. Fukuzumi, K. Ohkubo and T. Suenobu, Acc. Chem. Res., 2014, 47, 1455–1464 CrossRef CAS PubMed.
- (a) T. Miyatake and H. Tamiaki, Coord. Chem. Rev., 2010, 254, 2593–2602 CrossRef CAS; (b) S. Sengupta and F. Würthner, Acc. Chem. Res., 2013, 46, 2498–2512 CrossRef CAS PubMed; (c) M. R. Wasielewski, Acc. Chem. Res., 2009, 42, 1910–1921 CrossRef CAS PubMed; (d) T. S. Balaban, Acc. Chem. Res., 2005, 38, 612–623 CrossRef CAS PubMed.
- H.-J. Son, S. Jin, S. Patwardhan, S. J. Wezenberg, N. C. Jeong, M. So, C. E. Wilmer, A. A. Sarjeant, G. C. Schatz, R. Q. Snurr, O. K. Farha, G. P. Wiederrecht and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 862–869 CrossRef CAS PubMed.
- (a) A. Onoda, Y. Kakikura, T. Uematsu, S. Kuwabata and T. Hayashi, Angew. Chem., Int. Ed., 2012, 51, 2628–2631 CrossRef CAS; (b) K. Oohora, A. Onoda and T. Hayashi, Chem. Commun., 2012, 48, 11714–11726 RSC; (c) I. Cohen-Ofri, M. van Gastel, J. Grzyb, A. Brandis, I. Pinkas, W. Lubitz and D. Noy, J. Am. Chem. Soc., 2011, 133, 9526–9535 CrossRef CAS PubMed; (d) J. L. R. Anderson, C. T. Armstrong, G. Kodali, B. R. Lichtenstein, D. W. Watkins, J. A. Mancini, A. L. Boyle, T. A. Farid, M. P. Crump, C. C. Moser and P. L. Dutton, Chem. Sci., 2014, 5, 507–514 RSC; (e) F. A. Tezcan, B. R. Crane, J. R. Winkler and H. B. Gray, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 5002–5006 CrossRef CAS PubMed; (f) T. Koshiyama, M. Shirai, T. Hikage, H. Tabe, K. Tanaka, S. Kitagawa and T. Ueno, Angew. Chem., Int. Ed., 2011, 50, 4849–4852 CrossRef CAS PubMed; (g) H. Zemel and B. Hoffman, J. Am. Chem. Soc., 1981, 103, 1192–1201 CrossRef CAS; (h) A. Kuki and S. G. Boxer, Biochemistry, 1983, 22, 2923–2933 CrossRef CAS PubMed; (i) J. W. Springer, P. S. Parkes-Loach, K. R. Reddy, M. Krayer, J. Jiao, G. M. Lee, D. M. Niedzwiedzki, M. A. Harris, C. Kirmaier, D. F. Bocian, J. S. Lindsey, D. Holten and P. A. Loach, J. Am. Chem. Soc., 2012, 134, 4589–4599 CrossRef CAS PubMed.
- (a) R. A. Miller, N. Stephanopoulos, J. M. McFarland, A. S. Rosko, P. L. Geissler and M. B. Francis, J. Am. Chem. Soc., 2010, 132, 6068–6074 CrossRef CAS PubMed; (b) L. S. Witus and M. B. Francis, Acc. Chem. Res., 2011, 44, 774–783 CrossRef CAS PubMed; (c) Y. S. Nam, T. Shin, H. Park, A. P. Magyar, K. Choi, G. Fantner, K. A. Nelson and A. M. Belcher, J. Am. Chem. Soc., 2010, 132, 1462–1463 CrossRef CAS PubMed; (d) M. Endo, M. Fujitsuka and T. Majima, Chem. – Eur. J., 2007, 13, 8660–8666 CrossRef CAS PubMed.
- J.-H. Jeoung, D. A. Pippig, B. M. Martins, N. Wagener and H. Dobbek, J. Mol. Biol., 2007, 368, 1122–1131 CrossRef CAS PubMed.
- (a) T. Hayashi, in Handbook of Porphyrin Science, ed. K. M. Kadish, K. Smith and R. Guilard, World Scientific, Singapore, 2010, vol. 5, pp. 1–69 Search PubMed; (b) T. Hayashi, in Coordination Chemistry in Protein Cages: Principles, Design, and Applications, ed. T. Ueno, Y. Watanabe, John Wiley & Sons, Hoboken, NJ, 2013, pp. 87–110 Search PubMed.
- F. W. J. Teale, Biochim. Biophys. Acta, 1959, 35, 543 CrossRef CAS.
- The titration curve supports the 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of a ZnPP molecule into a heme pocket (Fig. S3, ESI†).
1 binding of a ZnPP molecule into a heme pocket (Fig. S3, ESI†). - The affinity of ZnPP to apoHTHP is approximately Ka > 106 M−1, because the characteristic UV-vis absorption spectrum of ZnPP bound in the HTHP matrices completely remains under highly diluted conditions (<1 μM).
- T. Komatsu, R.-M. Wang, P. A. Zunszain, S. Curry and E. Tsuchida, J. Am. Chem. Soc., 2006, 128, 16297–16301 CrossRef CAS PubMed.
- The apparent number of n is determined by the amount of apoHTHP added into a solution of rHTHPZnPP(6/6).
- Rapid decays within 20 ps by singlet–singlet annihilation were observed in only fully photosensitizer-inserted HTHPs (Table S2, Fig. S6 and S14, ESI†).
- The CD spectrum of rHTHPZnPP(1/6) shows only a positive Cotton effect in the region from 360 nm to 480 nm and is generally consistent with that of rHTHPZnPP(0.7/6), indicating a lack of cooperative binding of the cofactor molecules for adjacent monomers (Fig. S7, ESI†).
- (a) X. Huang, K. Nakanishi and N. Berova, Chirality, 2000, 12, 237–255 CrossRef CAS PubMed; (b) N. Berova, L. D. Bari and G. Pescitelli, Chem. Soc. Rev., 2007, 36, 914–931 RSC.
- Examples of a long-range exciton coupling: (a) S. Matile, N. Berova, K. Nakanishi, J. Fleischhauer and R. W. Woody, J. Am. Chem. Soc., 1996, 118, 5198–5206 CrossRef CAS; (b) K. Tsubaki, K. Takaishi, H. Tanaka, M. Miura and T. Kawabata, Org. Lett., 2006, 8, 2587–2590 CrossRef CAS PubMed.
- The decay components with lifetimes derived from electron transfer in the excited charge transfer complexes were not observed due to the instrument response (fwhm = ca. 1 ns).
- The energy migration lifetimes were estimated from the femtosecond transient absorption changes to be 0.69 ns and 0.54 ns for rHTHPZnPP(6/6) and rHTHPZnCe6(6/6), respectively (see details in Table S3, Fig. S12 and S16, ESI†).
- The difference between the binding constants of MV2+ for rHTHPZnCe6(n/6) and rHTHPZnPP(n/6) may be derived from the number of carboxylate groups of the cofactors.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc02680f |
| This journal is © The Royal Society of Chemistry 2015 |