 Open Access Article
Open Access ArticleDirect detection of the mercury–nitrogen bond in the thymine–HgII–thymine base-pair with 199Hg NMR spectroscopy†
Takenori
Dairaku‡
a,
Kyoko
Furuita‡
b,
Hajime
Sato‡
c,
Jakub
Šebera‡
de,
Daichi
Yamanaka
a,
Hiroyuki
Otaki
a,
Shoko
Kikkawa
a,
Yoshinori
Kondo
a,
Ritsuko
Katahira
b,
F.
Matthias Bickelhaupt
fg,
Célia
Fonseca Guerra
f,
Akira
Ono
h,
Vladimír
Sychrovský
*d,
Chojiro
Kojima
*b and
Yoshiyuki
Tanaka
*ai
aGraduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aza-Aoba, Aramaki, Aoba-ku, Sendai, Miyagi 980-8578, Japan. E-mail: tanaka@mail.pharm.tohoku.ac.jp
bInstitute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: kojima@protein.osaka-u.ac.jp
cApplication, Bruker BioSpin K.K., 3-9 Moriya-cho, Kanagawa-ku, Yokohama, Kanagawa 221-0022, Japan
dInstitute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, v.v.i., Flemingovo náměstí 2, 16610, Praha 6, Czech Republic. E-mail: vladimir.sychrovsky@uochb.cas.cz
eInstitute of Physics, Academy of Sciences of the Czech Republic, v.v.i, Na Slovance 2, CZ-182 21 Prague 8, Czech Republic
fDepartment of Theoretical Chemistry and Amsterdam Center for Multiscale Modeling (ACMM), VU University Amsterdam, De Boelelaan 1083, NL-1081 HV Amsterdam, The Netherlands
gInstitute for Molecules and Materials (IMM), Radboud University Nijmegen, Heyendaalseweg 135, NL-6525 AJ Nijmegen, The Netherlands
hDepartment of Material & Life Chemistry, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama, Kanagawa 221-8686, Japan
iFaculty of Pharmaceutical Sciences, Tokushima Bunri University, Yamashiro-cho, Tokushima 770-8514, Japan. E-mail: tanakay@ph.bunri-u.ac.jp
First published on 13th April 2015
Abstract
We have observed the 1-bond 199Hg–15N J-coupling (1J(199Hg,15N) = 1050 Hz) within the HgII-mediated thymine–thymine base pair (T–HgII–T). This strikingly large 1J(199Hg,15N) is the first one for canonical sp2-nitrogen atoms, which can be a sensitive structure-probe of N-mercurated compounds and a direct evidence for N-mercuration.
Mercury-199 NMR spectroscopy is used to probe coordination modes, coordinating elements, and the nature of metals in biomolecules.1 Within the 199Hg NMR data, those for N–Hg bonds are of particular importance as metals in proteins and in DNA/RNA molecules frequently interact with nitrogen atoms. Moreover, the N–HgII bond formation in the HgII-mediated thymine–thymine base pair (T–HgII–T) corresponds to an irregular “deprotonative” N-mercuration in water of a bulk proton source.2–7 In addition, extraordinary thermal stability with a positive reaction entropy was observed for N–HgII–N bonding in a DNA duplex.8–12 The stability of the Hg–DNA complex can be explained partly owing to the metallophilic attraction between Hg atoms in consecutive T–HgII–T base pairs, and the metallophilic attraction itself is a recent hot topic of inorganic chemistry.13–17
Despite such biological/chemical importance, N–HgII bonds remained uncharacterized. Particularly, the measurements of 1J(199Hg,15N) is challenging, owing to the large chemical shift anisotropy (CSA) of 199Hg and low natural abundance of 15N.18 The only |1J(199Hg,15N)| value of a linear two-coordinate complex was recorded for (Me3Si)2N–HgII–N(SiMe3)2.19 The |1J(199Hg,15N)| values for other coordination modes of 199Hg are also limited to HgII–CyDTA (trans-l,2-diaminocyclohexane-NNN′N′-tetraacetate)20 and HgII–(NHMe2)2Cl221 complexes (Tables S1 and S2 in ESI†). However, in all cases, some of important parameters such as structure, 15N or 199Hg NMR chemical shifts (δ(15N) or δ(199Hg)), 2-bond 15N–15N J-couplings across HgII, (2J(15N,15N)) or hybridization state of nitrogen atoms always remained unknown. Therefore, a complete 199Hg/15N NMR J/δ dataset for a structurally well-defined compound has never been recorded so far.
In this sense, the T–HgII–T base pair (Fig. 1) provides an excellent platform for studying 1J(199Hg,15N), as its chemical and 3-dimensional (3D) structures have been solidly determined3,6,7,12,22 and historically accumulated data2–4,23,24 are available. Regarding the NMR parameters of the T–HgII–T base pair, the 199Hg chemical shift δ(199Hg),23 the 2-bond 15N–15N J-coupling across HgII, 2J(15N,15N)6 and δ(15N)6 were previously determined. Hence, the only missing NMR parameter for characterizing the unique physicochemical properties of the N–HgII bond is 1J(199Hg,15N). Once it is measured for T–HgII–T, the T–HgII–T system will provide a complete J/δ dataset for 199Hg/15N with a reliable structure, and the 1J(199Hg,15N) value may provide a key concept for constructing molecular devices8,25–44 from HgII–DNA complexes.
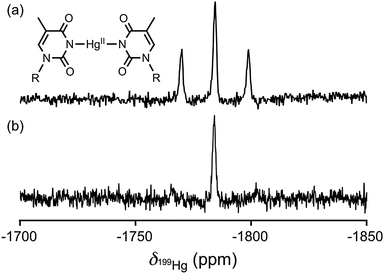 | ||
| Fig. 1 One-dimensional 199Hg NMR spectrum (71.667 MHz for 199Hg frequency) of the thymidine–HgII–thymidine complex (25 mM) in DMSO-d6 under natural abundance 199Hg (16.84%). (a) The 1D 199Hg NMR spectrum without 15N-decoupling. (b) The 1D 199Hg NMR spectrum with 15N-decoupling. The 199Hg NMR chemical shifts are displayed with respect to dimethylmercury (0 ppm) using 1 M HgCl2 in DMSO-d6 as a secondary reference (−1501 ppm).55 The chemical structure of the T–HgII–T is depicted above the spectrum, with “R” denoting ribose. | ||
To measure 1J(199Hg,15N) in T–HgII–T, its highly soluble 15N-labeled complex is crucial for 199Hg/15N signal detection. In addition, HgII–ligand exchanges must be suppressed to avoid the disappearance of 1J(199Hg,15N) owing to exchange broadening. Considering these facts, we determined the 1J(199Hg,15N) value by using a thymidine–HgII–thymidine complex (T–HgII–T). To confirm if the splitting of the 199Hg resonance is 1J(199Hg,15N), we monitored the disappearance of the splitting upon 15N-decoupling using a special NMR probe for detecting 15N-heteronucleus correlations. Lastly, the derived 1J(199Hg,15N) value was also investigated theoretically with relativistic density functional theory (DFT) including spin–orbit coupling effects.
In this study, we used 15N-labeled thymidine to produce 15N-labeled T–HgII–T. To suppress the exchange of HgII ligands, we prepared a sample that contained T–HgII–T exclusively, without any anion (competitive HgII–ligands against thymine). Such sample was prepared by the reaction [thymidine + HgO → T–HgII–T + H2O] followed by H2O evaporation.24 The resulting pure 15N-labeled T–HgII–T was subjected to 199Hg NMR measurements in dimethyl sulfoxide-d6 (DMSO-d6) (Fig. 1). The 199Hg NMR signal was successfully observed as a triplet resonance at δ(199Hg) = − 1784 ppm, with the absolute 1J-value |1J(199Hg,15N)| = 1050 Hz (Fig. 1 and Table 1). The observed δ(199Hg) value was the same as that observed previously in T–HgII–T,23 which ensured successful sampling.
| Ligand | Method | N-hybrida | |1JHgN|b | δ(199Hg)c |
|---|---|---|---|---|
| a Hybridization state of nitrogen atoms. b The “absolute” 1-bond 199Hg–15N J-coupling, |1J(199Hg,15N)|, in Hz. c 199Hg NMR chemical shift in ppm with respect to dimethylmercury (0 ppm). d The T–HgII–T complex. e The theoretical calculation (ZORA-SO-B3LYP/TZ2P) in this work. The average values of 1J(199Hg,15N) and δ(199Hg) were calculated for rotational conformers of thymidine–HgII–thymidine, because the energy barrier for rotation around the N–HgII–N axis was smaller than 1.1 kcal mol−1. The calculated δ(199Hg) and 1JHgN values were therefore averaged over respective rotamers (Table S5 in ESI). f The “–” sign was calculated for J-coupling (Table S4 in ESI). g The (Me3Si)2N–HgII–N(SiMe3)2 complex. h See Supporting discussion (ESI) for details. i Ref. 19. j Ref. 53. For chemical shift referencing see the footnote to Table S1 in ESI. It should be noted that 15N6 and 1H54 chemical shift perturbations for the thymidine–HgII–thymidine complexation were coherent with those observed for the formation of the T–HgII–T base-pairs in a DNA duplex (Table S1 in ESI). | ||||
| Thymined | Experiment | sp2 | 1050 | −1784 |
| Theorye | sp2 | 931f | −1848 | |
| N(SiMe3)2g | Experiment | sp2-likeh | 316.2i | −992j |
| Theorye | sp2-likeh | 278.4f | −827 | |
The 199Hg NMR spectrum under 15N-decoupling and 15N NMR spectrum were recorded to exclude the possibility that the observed splitting of the 199Hg signal might arise from a structural polymorphism. Notably, the splitting disappeared upon the 15N-decoupling (Fig. 1b). It should be further noted that this 15N-decoupled 199Hg NMR spectrum can't be recorded with conventionally available probes. This measurement became possible only by using the special probe, which can perform a 15N–199Hg double resonance spectroscopy. In addition, the splitting of the 15N resonance (1050 Hz) was observed as satellite peaks at δ(15N) = 184 ppm in the 1-dimensional 15N NMR spectrum (Fig. S1 in ESI†). Thus, the splitting of the 199Hg resonance shown in Fig. 1a should be interpreted as 1J(199Hg,15N).
The |1J(199Hg,15N)| value of 1050 Hz for T–HgII–T was strikingly larger than the 1J-coupling of (Me3Si)2N–HgII–N(SiMe3)2 (316.2 Hz),19 HgII–CyDTA complexes (365.7–395.5 Hz),20 and HgII–(NHMe2)2Cl2 (14.7 Hz)21 (Table 1 and Tables S1 and S2 in ESI†). Thus, the observed |1J(199Hg,15N)| value for T–HgII–T is the largest of all 1J-values reported to date.
Here we investigate the correlation between |1J(199Hg,15N)| value and N-hybridization state. Within the compounds whose |1J(199Hg,15N)| were reported, T–HgII–T and (Me3Si)2N–HgII–N(SiMe3)2 possess the linear two-coordinate structure, and their |1J(199Hg,15N)| values can be compared. Regarding the N-hybridization state of (Me3Si)2N–HgII–N(SiMe3)2, an sp2-like planar structure of the nitrogen atoms was suggested from the electron diffraction study,19 which is further supported by Bent's rule45 (see Supporting discussion in ESI† for Bent's rule). Therefore, the HgII-bound nitrogen atoms in both samples belong to the sp2 category basically, and the current data of |1J(199Hg,15N)| are insufficient for us to correlate between |1J(199Hg,15N)| and N-hybridization, due to the lack of the 1J-values for N(sp)–HgII and N(sp3)–HgII bonds.
As a further investigation, |1J(199Hg,15N)| values for the “sp2 nitrogen” in T–HgII–T and “sp2-like nitrogen” in (Me3Si)2N–HgII–N(SiMe3)2 were strikingly different (Table 1). However, this may be because the sp2-like N-hybridization in (Me3Si)2N–HgII–N(SiMe3)2 might be different from the “canonical sp2 nitrogen” in T–HgII–T. This possibility was also inferred from 14/15N NMR spectroscopic data,46 where the 14N NMR chemical shift for the Si2N–HgII–NSi2 linkage showed a rather sp3-like value (δ(14N) = 66.2 ppm,46 Table S1 in ESI†). By contrast, δ(15N) for HgII-linked N3 in T–HgII–T is 184 ppm, and the value is located within the empirical range for an sp2-hybridized nitrogen (Fig. S1 and Table S1 in ESI†). From these facts, the N-hybridization state of (Me3Si)2N–HgII–N(SiMe3)2 can't be unambiguously assigned (see also Supporting discussion in ESI† for details). However, on the basis of the investigations mentioned above, the |1J(199Hg,15N)| value might be a sensitive NMR parameter for detecting differences in the fine electronic structures of T–HgII–T and (Me3Si)2N–HgII–N(SiMe3)2.
We then quantum chemically computed |1J(199Hg,15N)| = 931 Hz and δ(199Hg) = − 1848 ppm for T–HgII–T using DFT including relativistic corrections from the zeroth-order regular approximation (ZORA) with spin–orbit (SO) coupling, as implemented in the ADF program47–49 (see Table 1 and Tables S3–S5 in ESI†). The theoretical |1J(199Hg,15N)| and δ(199Hg) values agree well with the experimental data (Table 1). With reference to the theoretical values given by Bagno and Saielli (|1J(199Hg,15N)| = 670 Hz, δ(199Hg) = − 1727 ppm),50 the theoretical |1J(199Hg,15N)| value was refined by using the complex where it was actually recorded (Table S3 in ESI†). The |1J(199Hg,15N)| value of 278.4 Hz calculated for (Me3Si)2N–HgII–N(SiMe3)2 also agreed satisfactorily with experiment (316.2 Hz).19 The signs of 1J(199Hg,15N) for T–HgII–T and (Me3Si)2N–HgII–N(SiMe3)2 were both “–” theoretically (Table S4 in ESI†).
In order to investigate the correlation between |1J(199Hg,15N)| values and N-hybridization states theoretically, we further analyzed the theoretical 1J(199Hg,15N). The calculated 1J(199Hg,15N) values were dependent on the “Fermi Contact” + “Spin Dipole coupling” (FC + SD) term (Table S4 in ESI†). With the dominance of this FC term, one may find the correlation between the N-hybridization and |1J(199Hg,15N)| in the future, although it should be experimentally explored.
Empirically, the δ(199Hg) values are clustered in terms of linked elements, hybridization states, and other factors of HgII-linked atoms (Table S2 in ESI†). Such phenomena were explained on the basis of the empirical correlation of δ(199Hg) with the ionicity of the X–HgII bond (high ionicity → up-field shift of δ(199Hg)),51 Unfortunately, owing to both a paucity of experimental δ(199Hg) values for a linear two-coordinate N–HgII–N linkage and the uncertain N-hybridization state in (Me3Si)2N–HgII–N(SiMe3)2, the correlation between δ(199Hg) and the N-hybridization state of HgII-linked nitrogen also remains obscure. Nevertheless, the highly up-field-shifted δ(199Hg) value for T–HgII–T among those of N-mercurated compounds suggests that N(sp2)–HgII–N(sp2) covalent linkages possess significant ionic character, which agrees with our previous studies (Table 1 and Tables S1 and S2 in ESI†).7,52 This observation suggests that δ(199Hg) values can be used as a sensitive indicator for probing the HgII coordination environment not only in C-mercurated complexes but also in N-mercurated complexes, including metalloproteins1 and metallo-DNA/RNA.
Accordingly, 199Hg NMR parameters, especially 1J(199Hg,15N), are sensitive parameters for characterizing the electronic structures of N-mercurated complexes and their N–HgII bonds as well as their Hg atoms. Hence, the 1J(199Hg,15N) value could be a key parameter for predicting the physicochemical properties of N-mercurated complexes and making them into molecular devices, based on a bottom-up approach.
The |1J(199Hg,15N)| value of 1050 Hz has been reported for canonical sp2-hybridized nitrogen for the first time. From this result, the T–HgII–T system provides a comprehensive and reliable 199Hg/15N NMR dataset for probing the HgII environment in N-mercurated compounds. This newly observed 1J(199Hg,15N) coupling can be used for detecting N–Hg bond formations and precisely characterizing these bonds.
This work was performed using the NMR spectrometer under the Cooperative Research Program of the Institute for Protein Research, Osaka University. This work was supported by the Platform for Drug Discovery, Informatics, and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This work was supported by grants-in-aid for Scientific Research (A) (24245037 to A.O. and Y.T), (B) (24310163 to Y.T and C.K.), (C) (18550146 to Y.T) from MEXT, Japan; a Human Frontier Science Program (HFSP) Young Investigator Grant from HFSPO, France (Y.T. and V.S.); and GAČR (P205/10/0228 and 16-12465S to V.S.) from the Czech Republic. T.D. and K.F. are the recipients of a Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science (JSPS). Y.T. and V.S. were further supported by an Invitation Fellowship for Research in Japan (Short-Term) from JSPS. F.M.B. and C.F.G. were supported by the National Research School Combination – Catalysis (NRSC-C) and the Netherlands Organization for Scientific Research (NWO-CW and NWO-EW).
Notes and references
- L. M. Utschig, J. W. Bryson and T. V. O’Halloran, Science, 1995, 268, 380–385 CAS.
- S. Katz, Biochim. Biophys. Acta, 1963, 68, 240–253 CrossRef CAS ; references cited therein.
- L. D. Kosturko, C. Folzer and R. F. Stewart, Biochemistry, 1974, 13, 3949–3952 CrossRef CAS.
- Z. Kuklenyik and L. G. Marzilli, Inorg. Chem., 1996, 35, 5654–5662 CrossRef CAS PubMed.
- Y. Miyake, H. Togashi, M. Tashiro, H. Yamaguchi, S. Oda, M. Kudo, Y. Tanaka, Y. Kondo, R. Sawa, T. Fujimoto, T. Machinami and A. Ono, J. Am. Chem. Soc., 2006, 128, 2172–2173 CrossRef CAS PubMed.
- Y. Tanaka, S. Oda, H. Yamaguchi, Y. Kondo, C. Kojima and A. Ono, J. Am. Chem. Soc., 2007, 129, 244–245 CrossRef CAS PubMed.
- T. Uchiyama, T. Miura, H. Takeuchi, T. Dairaku, T. Komuro, T. Kawamura, Y. Kondo, L. Benda, V. Sychrovský, P. Bouř, I. Okamoto, A. Ono and Y. Tanaka, Nucleic Acids Res., 2012, 40, 5766–5774 CrossRef CAS PubMed.
- A. Ono and H. Togashi, Angew. Chem., 2004, 116, 4400–4402 ( Angew. Chem., Int. Ed. , 2004 , 43 , 4300–4302 ) CrossRef PubMed.
- H. Torigoe, A. Ono and T. Kozasa, Chem. – Eur. J., 2010, 16, 13218–13225 CrossRef CAS PubMed.
- H. Torigoe, Y. Miyakawa, A. Ono and T. Kozasa, Thermochim. Acta, 2012, 532, 28–35 CrossRef CAS PubMed.
- J. Šebera, J. Burda, M. Straka, A. Ono, C. Kojima, Y. Tanaka and V. Sychrovský, Chem. – Eur. J., 2013, 19, 9884–9894 CrossRef PubMed.
- H. Yamaguchi, J. Šebera, J. Kondo, S. Oda, T. Komuro, T. Kawamura, T. Daraku, Y. Kondo, I. Okamoto, A. Ono, J. V. Burda, C. Kojima, V. Sychrovský and Y. Tanaka, Nucleic Acids Res., 2014, 42, 4094–4099 CrossRef CAS PubMed.
- P. Pyykkö, Chem. Rev., 1997, 97, 597–636 CrossRef PubMed.
- F.-A. Polonius and J. Müller, Angew. Chem., 2007, 119, 5698–5701 ( Angew. Chem., Int. Ed. , 2007 , 46 , 5602–5604 ) CrossRef PubMed.
- L. Benda, M. Straka, Y. Tanaka and V. Sychrovský, Phys. Chem. Chem. Phys., 2011, 13, 100–103 RSC.
- L. Benda, M. Straka, V. Sychrovský, P. Bouř and Y. Tanaka, J. Phys. Chem. A, 2012, 116, 8313–8320 CrossRef CAS PubMed.
- S. Kumbhar, S. Johannsen, R. K. O. Sigel, M. P. Waller and J. Müller, J. Inorg. Biochem., 2013, 127, 203–210 CrossRef CAS PubMed.
- J. Mason and R. J. Goodfellow, in Multinuclear NMR, ed. J. Mason, Plenum Press, New York, 1987, ch. 12 & 21 Search PubMed.
- P. Bernatowicz, S. Szymański and B. Wrackmeyer, J. Phys. Chem. A, 2001, 105, 6414–6419 CrossRef CAS.
- E. H. Curzon, N. Herron and P. Moore, J. Chem. Soc., Dalton Trans., 1980, 721–725 RSC.
- S. S. Al-Showiman, Inorg. Chim. Acta, 1988, 141, 263–274 CrossRef CAS.
- J. Kondo, T. Yamada, C. Hirose, I. Okamoto, Y. Tanaka and A. Ono, Angew. Chem., 2014, 126, 2417–2420 ( Angew. Chem., Int. Ed. , 2014 , 53 , 2385–2388 ) CrossRef PubMed.
- A. R. Norris and R. Kumar, Inorg. Chim. Acta, 1984, 93, 33–35 CrossRef CAS.
- E. Buncel, C. Boone, H. Joly, R. Kumar and A. R. Norris, J. Inorg. Biochem., 1985, 25, 61–73 CrossRef CAS.
- K. Tanaka and M. Shionoya, J. Org. Chem., 1999, 64, 5002–5003 CrossRef CAS.
- H. Weizman and Y. Tor, J. Am. Chem. Soc., 2001, 123, 3375–3376 CrossRef CAS.
- C. Switzer, S. Sinha, P. H. Kim and B. D. Heuberger, Angew. Chem., 2005, 117, 1553–1556 ( Angew. Chem., Int. Ed. , 2005 , 44 , 1529–1532 ) CrossRef PubMed.
- K. Tanaka, G. H. Clever, Y. Takezawa, Y. Yamada, C. Kaul, M. Shionoya and T. Carell, Nat. Nanotechnol., 2006, 1, U190–U195 CrossRef PubMed.
- G. H. Clever, C. Kaul and T. Carell, Angew. Chem., 2007, 119, 6340–6350 ( Angew. Chem., Int. Ed. , 2007 , 46 , 6226–6236 ) CrossRef PubMed.
- J. Müller, Eur. J. Inorg. Chem., 2008, 3749–3763 CrossRef PubMed.
- A. Ono, S. Cao, H. Togashi, M. Tashiro, T. Fujimoto, T. Machinami, S. Oda, Y. Miyake, I. Okamoto and Y. Tanaka, Chem. Commun., 2008, 4825–4827 RSC.
- S. Johannsen, N. Megger, D. Böhme, R. K. O. Sigel and J. Müller, Nat. Chem., 2010, 2, 229–234 CrossRef CAS PubMed.
- A. Ono, H. Torigoe, Y. Tanaka and I. Okamoto, Chem. Soc. Rev., 2011, 40, 5855–5866 RSC.
- E. Meggers, P. L. Holland, W. B. Tolman, F. E. Romesberg and P. G. Schultz, J. Am. Chem. Soc., 2000, 122, 10714–10715 CrossRef CAS.
- S. Atwell, E. Meggers, G. Spraggon and P. G. Schultz, J. Am. Chem. Soc., 2001, 123, 12364–12367 CrossRef CAS PubMed.
- E. Meggers, Curr. Opin. Chem. Biol., 2007, 11, 287–292 CrossRef CAS PubMed.
- M. K. Schlegel, L.-O. Essen and E. Meggers, J. Am. Chem. Soc., 2008, 130, 8158–8159 CrossRef CAS PubMed.
- K. Tanaka, A. Tengeiji, T. Kato, N. Toyama and M. Shionoya, Science, 2003, 299, 1212–1213 CrossRef CAS PubMed.
- G. H. Clever, S. J. Reitmeier, T. Carell and O. Schiemann, Angew. Chem., 2010, 122, 5047–5049 ( Angew. Chem., Int. Ed. , 2010 , 49 , 4927–4929 ) CrossRef PubMed.
- T. Carell, C. Behrens and J. Gierlich, Org. Biomol. Chem., 2003, 1, 2221–2228 CAS.
- T. Ito, G. Nikaido and S. I. Nishimoto, J. Inorg. Biochem., 2007, 101, 1090–1093 CrossRef CAS PubMed.
- J. Joseph and G. B. Schuster, Org. Lett., 2007, 9, 1843–1846 CrossRef CAS PubMed.
- L. Q. Guo, N. Yin and G. N. Chen, J. Phys. Chem. C, 2011, 115, 4837–4842 CAS.
- H. Isobe, N. Yamazaki, A. Asano, T. Fujino, W. Nakanishi and S. Seki, Chem. Lett., 2011, 40, 318–319 CrossRef CAS.
- H. A. Bent, Chem. Rev., 1961, 61, 275–311 CrossRef CAS.
- O. Just, D. A. Gaul and W. S. Rees, Jr., Polyhedron, 2001, 20, 815–821 CrossRef CAS.
- G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931–967 CrossRef CAS PubMed.
- M. Swart, C. Fonseca Guerra and F. M. Bickelhaupt, J. Am. Chem. Soc., 2004, 126, 16718–16719 CrossRef CAS PubMed.
- J. M. Fonville, M. Swart, Z. Vokacova, V. Sychrovský, J. E. Šponer, J. Šponer, C. W. Hilbers, F. M. Bickelhaupt and S. S. Wijmenga, Chem. – Eur. J., 2012, 18, 12372–12387 CrossRef CAS PubMed.
- A. Bagno and G. Saielli, J. Am. Chem. Soc., 2007, 129, 11360–11361 CrossRef CAS PubMed.
- D. Rehder, Coord. Chem. Rev., 1991, 110, 161–210 CrossRef CAS.
- Y. Tanaka and A. Ono, Dalton Trans., 2008, 4965–4974 RSC.
- A. E. Wetherby, Jr., S. D. Benson and C. S. Weinert, Inorg. Chim. Acta, 2007, 360, 1977–1986 CrossRef PubMed.
- Y. Tanaka, H. Yamaguchi, S. Oda, M. Nomura, C. Kojima, Y. Kondo and A. Ono, Nucleosides, Nucleotides Nucleic Acids, 2006, 25, 613–624 CAS.
- S. S. Lemos, D. U. Martins, V. M. Deflon and J. Elena, J. Organomet. Chem., 2009, 694, 253–258 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional information as noted in text. See DOI: 10.1039/c5cc02423d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |
