Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient masking of corrosion and fission products such as Ni(II) and Pd(II) in the presence of the minor actinide Am(III) using hydrophilic anionic or cationic bis-triazines†
Frank W.
Lewis
*ab,
Laurence M.
Harwood
*a,
Michael J.
Hudson
a,
Udo
Müllich
c and
Andreas
Geist
c
aDepartment of Chemistry, The University of Reading, Whiteknights, Reading RG6 6AD, UK. E-mail: l.m.harwood@reading.ac.uk
bDepartment of Applied Sciences, Faculty of Health and Life Sciences, Northumbria University, Newcastle Upon Tyne NE1 8ST, UK. E-mail: frank.lewis@northumbria.ac.uk
cKarlsruher Institut für Technologie (KIT-INE), Institut für Nukleare Entsorgung, Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany. E-mail: andreas.geist@kit.edu
First published on 1st May 2015
Abstract
Water soluble anionic and cationic bis-triazine ligands are able to suppress (mask) the extraction of corrosion and fission products such as Ni(II) and Pd(II) that are found in PUREX raffinates. Thus it is possible to separate these elements from the minor actinide Am(III). Although some masking agents have previously been developed that retard the extraction of Pd(II), this is the first time a masking agent has been developed for Ni(II).
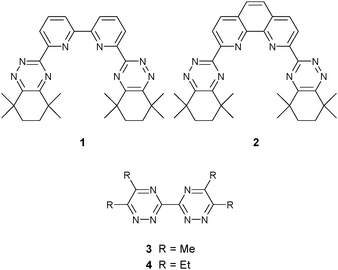
After the removal of plutonium and uranium in the PUREX process, the dominant part of the long-term radiotoxicity and heat load of spent nuclear fuels arises from minor actinides such as americium. Separation of the minor actinides, which include Am, Cm and Np, from fission products such as the lanthanides by solvent extraction has been achieved using soft N-donor molecules containing the 1,2,4-triazine moiety.1–7 Molecules such as the quadridentate ligands CyMe4-BTBP 18,9 and CyMe4-BTPhen 210–12 (Fig. 1) are able to extract and separate the minor actinides [Am(III), Cm(III)] from the lanthanides with very high selectivities. Indeed, CyMe4-BTBP 1 is the current European reference extraction reagent for further development of the SANEX (Selective ActiNide Extraction) process.13 In this process the radionuclides and non-fissile fission products are in an aqueous nitric acid phase with the extraction reagent in an organic solvent. Furthermore, CyMe4-BTBP 1 is also being studied both for the direct selective extraction of Am(III) and Cm(III),14–17 and for the co-extraction of all trans-uranic elements directly from PUREX raffinate.18 Unfortunately, CyMe4-BTBP 1 not only co-extracts the minor actinides in these processes but certain corrosion and fission products such as Ni(II), Pd(II), Ag(I) and Cd(II) and their presence in the waste stream can interfere with the extraction protocol. The solid state structures of some of these [M(CyMe4-BTBP)n]m+ complexes have been recently reported.19
Although much progress has been made concerning the partitioning of the minor actinides from the lanthanides,1–7 far less progress has been made on the separation of the minor actinides from corrosion and fission products such as Ni(II), Pd(II), Ag(I) and Cd(II). One way to separate these elements from the minor actinides could be to hold the fission products in the aqueous phase during the selective extraction of the actinides by CyMe4-BTBP 1 into the organic phase by using a hydrophilic complexing agent. This new reagent in the aqueous phase would need to be able to complex the corrosion and fission products selectively without complexing either the minor actinides or lanthanides. Such reagents could also be useful in conventional hydrometallurgy as selective complexing agents for certain precious metals. In addition, the hydrophilic reagent needs to be able to form water soluble complexes. L-Cysteine14–17 and a chelating agent derived from methionine20 have previously been used as selective complexing or ‘masking’ agents for Pd(II), while trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid (CDTA) can prevent the extraction of Pd(II) and Zr(IV) by hydrophobic O-donor ligands.21,22 However, no reagents have been reported to date that can mask a range of corrosion and fission products.
It has been shown, however, that bidentate molecules containing the 1,2,4-triazine moiety do not interact significantly with Am(III) or with the trivalent lanthanides [Ln(III)].23 We considered, therefore, that hydrophilic bidentate 3,3′-bis(1,2,4-triazine) ligands could be capable of masking several fission and corrosion products based on a series of observations. Hydrophobic 3,3′-bis(1,2,4-triazine) ligands such as 3 and 4 (Fig. 1) form strong complexes with several late transition metals such as Fe(II), Co(II), Ni(II), Cu(II), Ru(II) and Pt(II).24–29 Furthermore, it has been observed that hydrophobic 3,3′-bis(1,2,4-triazine) ligands do not extract or separate Am(III) or Eu(III).23 Indeed, ligand 4 has been used as a phase-transfer agent in the separation of Am(III) from Eu(III) by the BTBPs but 4 does not itself extract either element.30 We thus proposed that hydrophilic 3,3′-bis(1,2,4-triazine) molecules could be able to complex several corrosion and fission products and prevent their extraction by CyMe4-BTBP 1, without complexing either Am(III) or Ln(III). In this communication, we investigate the ability of two new hydrophilic 3,3′-bis(1,2,4-triazine) molecules to mask some problematic corrosion and fission products that are co-extracted from the PUREX raffinate by CyMe4-BTBP 1 using Ni(II), Pd(II), Ag(I) and Cd(II) as exemplars for a wider range of elements.
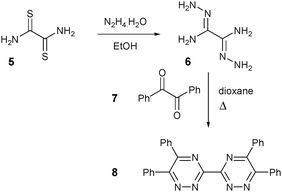
The hydrophilic 3,3′-bis(1,2,4-triazines) chosen for consideration were molecules 10 and 13. The former is a sulfonic acid derivative and the latter has quaternary ammonium groups. The novel tetrasulfonated 3,3′-bis(1,2,4-triazine) molecule 10 was synthesized as shown in Schemes 1 and 2. Intermediate 8 was synthesized from dithiooxamide 5 in two steps according to the literature (Scheme 1).23,27
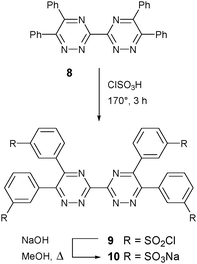
Sulfonation of 8 with chlorosulfonic acid afforded the tetrasulfonyl chloride 9 and hydrolysis of 9 with sodium hydroxide in methanol gave the desired tetrasodium sulfonate 3,3′-bis(1,2,4-triazine) ligand 10 (Scheme 2).
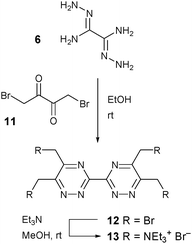
The tetra quaternary ammonium salt 3,3′-bis(1,2,4-triazine) ligand 13 was synthesized as shown in Scheme 3. Intermediate 12 was synthesized from 6 and 11 according to the literature procedure31 and subsequently reacted with triethylamine in methanol to afford the desired hydrophilic ligand 13 (Scheme 3).
Initial extraction experiments were performed in order to judge whether the molecules 10 and 13 had any potential for masking the above fission and corrosion products that would otherwise be extracted by CyMe4-BTBP 1 into 1-octanol. Neither masking agent had any significant influence on the extraction or separation of Am(III) and Eu(III) by CyMe4-BTBP 1. Ni(II) extraction by 1 was suppressed both by 10 and 13. Pd(II) extraction by 1 was suppressed by 13. Ag(I) extraction by 1 seems to be suppressed by both 10 and 13 but the results were inconclusive. No results could be obtained for Cd(II) which was below the detection limit in all aqueous samples. Therefore, Cd(II) extraction by CyMe4-BTBP 1 was not suppressed by either masking agent. This is not surprising, as the BTBPs are quadridentate ligands and are thus stronger ligands toward Cd(II) than the bidentate ligands 10 and 13.
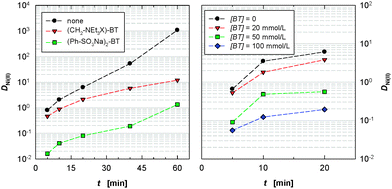
More detailed experiments were then performed by varying the contact-time in the extraction experiments and adding a second extracting agent, N,N,N′,N′-tetraoctyldiglycolamide (TODGA), which is used to accelerate the rate of actinide extraction by CyMe4-BTBP 1.17 As shown in Fig. 2 left, CyMe4-BTBP 1 extracts Ni(II) slowly but with high distribution ratios (D) in the absence of a masking agent. For contacting times of several minutes, DNi(II) ≈ 1, meaning that approximately 50% is extracted. However, once extracted, Ni(II) is difficult to remove from the organic phase since its distribution ratio is greater than 1000 at equilibrium.
Addition of reagent 10 (20 mM) efficiently suppresses Ni(II) extraction (DNi(II) < 0.10) by 1 even for longer contacting times of up to 20 minutes (Fig. 2 left). It is notable that, although some masking agents have previously been developed that retard the extraction of Pd(II),17,32 none have so far been developed that can retard the extraction of Ni(II).
Adding 13 (20 mM) to the aqueous phase suppresses Ni(II) extraction by 1 but not to a large extent (after 10 min, DNi(II) ≈ 1). We then examined the ability of 13 to mask Ni(II) at different concentrations since it would be preferred over the sulfur-containing 10 from the viewpoint of high level waste treatment as the raffinate solution containing the corrosion and fission products and masking agent will be ultimately incinerated and vitrified following the separation process. Molecule 13 could be fully incinerated to innocuous gases if the bromide ion is exchanged for nitrate, whereas 10 would generate acidic waste on incineration owing to its sulfur content. As shown in Fig. 2 right, 13 does suppress the extraction of Ni(II) to a useful extent (at 100 mM 13, DNi(II) ≈ 0.1 for contacting times of 5–10 minutes).
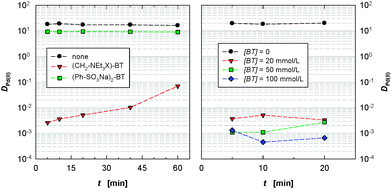
In the absence of a masking agent, Pd(II) is extracted rapidly by CyMe4-BTBP 1 (equilibrium is attained within <5 minutes with DPd(II) ≈ 20, Fig. 3 left). The strong attraction by CyMe4-BTBP 1 for Pd(II) arises from the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 square-planar complex with Pd(II).19 Adding 10 (20 mM) to the aqueous phase slightly lowers Pd(II) extraction by 1 (DPd(II) ≈ 10) without affecting the extraction kinetics. On the other hand, addition of 13 (20 mM) to the aqueous phase efficiently suppresses Pd(II) extraction by CyMe4-BTBP 1 (for contacting times up to 40 min, DPd(II) < 0.01). A slow increase in Pd(II) extraction was observed with contact time indicating that 13 masks Pd(II) kinetically. However, for practical reasons Pd(II) extraction is efficiently suppressed by 13 during the contact times typically used in waste treatment. Increasing the concentration of 13 further suppresses the extraction of Pd(II) by CyMe4-BTBP 1 (Fig. 3 right). However, owing to the low concentrations of Pd(II) in the aqueous phase the D values are not precise (D values below 0.1 have errors up to ±20%).
1 square-planar complex with Pd(II).19 Adding 10 (20 mM) to the aqueous phase slightly lowers Pd(II) extraction by 1 (DPd(II) ≈ 10) without affecting the extraction kinetics. On the other hand, addition of 13 (20 mM) to the aqueous phase efficiently suppresses Pd(II) extraction by CyMe4-BTBP 1 (for contacting times up to 40 min, DPd(II) < 0.01). A slow increase in Pd(II) extraction was observed with contact time indicating that 13 masks Pd(II) kinetically. However, for practical reasons Pd(II) extraction is efficiently suppressed by 13 during the contact times typically used in waste treatment. Increasing the concentration of 13 further suppresses the extraction of Pd(II) by CyMe4-BTBP 1 (Fig. 3 right). However, owing to the low concentrations of Pd(II) in the aqueous phase the D values are not precise (D values below 0.1 have errors up to ±20%).
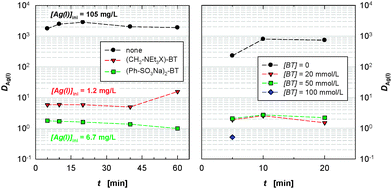
In the absence of a masking agent, Ag(I) is rapidly extracted by CyMe4-BTBP 1 (DAg(II) > 1000, Fig. 4 left). Both masking agents 10 and 13 suppress the extraction of Ag(I) by CyMe4-BTBP 1 to some extent (with 20 mM 10, DAg(I) < 2, with 20 mM 13, DAg(I) ≈ 6). However, both masking agents also precipitate some Ag(I), as seen from the formation of a precipitate upon adding the masking agent to the aqueous phase and from the measured Ag concentrations in the aqueous samples before extraction. The D values shown in Fig. 4 are based on the fraction of Ag(I) remaining in solution. In the case of 13, Ag(I) is precipitated as AgBr in an ion exchange reaction. Fig. 4 right shows the effect of adding varied concentrations of 13 on the extraction of Ag(I) by CyMe4-BTBP 1.
In summary, we report the first examples of hydrophilic 3,3′-bis(1,2,4-triazine) ligands, and their application as masking agents in order to suppress the extraction of certain problematic corrosion and fission products that are to be found in nitric acid solutions of PUREX raffinates. The bidentate tetrasulfonated ligand 10 efficiently suppresses the extraction of Ni(II) by solutions of CyMe4-BTBP 1. Interestingly, 10 has little effect on the suppression of Pd(II) extraction by 1. The reasons for the different complexation behavior of the ligands towards Ni(II) and Pd(II) are unclear at this point, and will be the subject of further ongoing studies in our laboratory. Neither masking agent is able to prevent the extraction of Cd(II), which is known to be strongly extracted by solutions of CyMe4-BTBP 1 and TODGA.14–17 We propose that these water soluble, bidentate triazine ligands warrant further study as masking agents. They could find applications in future analytical or industrial separations as versatile masking agents for the removal of several fission and corrosion products.
We acknowledge Financial support from the Commission of the European Community under the ACSEPT (FP7-CP-2007-211267) and SACSESS (FP7-CP-2012-323282) projects. We also gratefully acknowledge the use of the Chemical Analysis Facility at the University of Reading.
Notes and references
- F. W. Lewis, M. J. Hudson and L. M. Harwood, Synlett, 2011, 2609–2632 CAS.
- M. J. Hudson, L. M. Harwood, D. M. Laventine and F. W. Lewis, Inorg. Chem., 2013, 52, 3414–3428 CrossRef CAS PubMed.
- M. J. Hudson, F. W. Lewis and L. M. Harwood, in Strategies and Tactics in Organic Synthesis, ed. M. Harmata, Elsevier, Amsterdam, 2013, vol. 9, pp. 177–202 Search PubMed.
- P. J. Panak and A. Geist, Chem. Rev., 2013, 113, 1199–1236 CrossRef CAS PubMed.
- J.-H. Lan, W.-Q. Shi, L.-Y. Yuan, Y.-L. Zhao, J. Li and Z.-F. Chai, Inorg. Chem., 2011, 50, 9230–9237 CrossRef CAS PubMed.
- C.-L. Xiao, Q.-Y. Wu, C.-Z. Wang, Y.-L. Zhao, Z.-F. Chai and W.-Q. Shi, Inorg. Chem., 2014, 53, 10846–10853 CrossRef CAS PubMed.
- J.-H. Lan, W.-Q. Shi, L.-Y. Yuan, J. Li, Y.-L. Zhao and Z.-F. Chai, Coord. Chem. Rev., 2012, 256, 1406–1417 CrossRef CAS PubMed.
- A. Geist, C. Hill, G. Modolo, M. R. St. J. Foreman, M. Weigl, K. Gompper, M. J. Hudson and C. Madic, Solvent Extr. Ion Exch., 2006, 24, 463–483 CrossRef CAS PubMed.
- D. Magnusson, B. Christiansen, M. R. S. Foreman, A. Geist, J.-P. Glatz, R. Malmbeck, G. Modolo, D. Serrano-Purroy and C. Sorel, Solvent Extr. Ion Exch., 2009, 27, 97–106 CrossRef CAS PubMed.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, J. F. Desreux, G. Vidick, N. Bouslimani, G. Modolo, A. Wilden, M. Sypula, T.-H. Vu and J.-P. Simonin, J. Am. Chem. Soc., 2011, 133, 13093–13102 CrossRef CAS PubMed.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, V. Hubscher-Bruder, V. Videva, F. Arnaud-Neu, K. Stamberg and S. Vyas, Inorg. Chem., 2013, 52, 4993–5005 CrossRef CAS PubMed.
- A. Afsar, L. M. Harwood, M. J. Hudson, P. Distler and J. John, Chem. Commun., 2014, 50, 15082–15085 RSC.
- S. Bourg, C. Hill, C. Caravaca, C. Rhodes, C. Ekberg, R. Taylor, A. Geist, G. Modolo, L. Cassayre, R. Malmbeck, M. Harrison, G. de Angelis, A. Espartero, S. Bouvet and N. Ouvrier, Nucl. Eng. Des., 2011, 241, 3427–3435 CrossRef CAS PubMed.
- A. Wilden, C. Schreinemachers, M. Sypula and G. Modolo, Solvent Extr. Ion Exch., 2011, 29, 190–212 CrossRef CAS PubMed.
- D. Magnusson, A. Geist, A. Wilden and G. Modolo, Solvent Extr. Ion Exch., 2013, 31, 1–11 CrossRef CAS PubMed.
- A. Wilden, G. Modolo, C. Schreinemachers, F. Sadowski, S. Lange, M. Sypula, D. Magnusson, A. Geist, F. W. Lewis, L. M. Harwood and M. J. Hudson, Solvent Extr. Ion Exch., 2013, 31, 519–537 CrossRef CAS PubMed.
- G. Modolo, A. Wilden, H. Daniels, A. Geist, D. Magnusson and R. Malmbeck, Radiochim. Acta, 2013, 101, 155–162 CrossRef CAS.
- E. Aneheim, C. Ekberg, M. R. S. Foreman, E. Lofstrom-Engdahl and N. Mabile, Sep. Sci. Technol., 2012, 47, 663–669 CrossRef CAS PubMed.
- E. Aneheim, B. Grüner, C. Ekberg, M. R. St. J. Foreman, Z. Hájková, E. Löfström-Engdahl, M. G. B. Drew and M. J. Hudson, Polyhedron, 2013, 50, 154–163 CrossRef CAS PubMed.
- E. Aneheim, C. Ekberg and M. R. St. J. Foreman, Hydrometallurgy, 2012, 115–116, 71–76 CrossRef CAS PubMed.
- M. Sypula, A. Wilden, C. Schreinemachers, R. Malmbeck, A. Geist, R. Taylor and G. Modolo, Solvent Extr. Ion Exch., 2012, 30, 748–764 CrossRef CAS PubMed.
- P. K. Nayak, R. Kumaresan, S. Chaurasia, K. A. Venkatesan, G. G. S. Subramanian, S. Rajeswari, M. P. Antony, P. R. Vasudeva Rao and B. M. Bhanage, Radiochim. Acta, 2015, 103, 359–368 CAS.
- M. J. Hudson, M. R. St. J. Foreman, C. Hill, N. Huet and C. Madic, Solvent Extr. Ion Exch., 2003, 21, 637–652 CrossRef CAS.
- R. E. Jensen and R. T. Pflaum, Anal. Chim. Acta, 1965, 32, 235–244 CrossRef CAS.
- J. Breu, K.-J. Range and E. Herdtweck, Monatsh. Chem., 1994, 125, 119–140 CrossRef CAS.
- V. Maheshwari, P. A. Marzilli and L. G. Marzilli, Inorg. Chem., 2008, 47, 9303–9313 CrossRef CAS PubMed.
- Y. Chen, X. Zhou, X.-H. Wei, B.-L. Yu, H. Chao and L.-N. Ji, Inorg. Chem. Commun., 2010, 13, 1018–1020 CrossRef CAS PubMed.
- F.-R. Dai, Y.-H. Wu, L.-Y. Zhang, B. Li, L.-X. Shi and Z.-N. Chen, Eur. J. Inorg. Chem., 2011, 2306–2316 CrossRef CAS PubMed.
- S. Roy, T. Blane, A. Lilio and C. P. Kubiak, Inorg. Chim. Acta, 2011, 374, 134–139 CrossRef CAS PubMed.
- J. Narbutt and J. Krejzler, Radiochim. Acta, 2008, 96, 219–223 CrossRef CAS.
- C. Yang and Y.-D. Cui, Synth. Commun., 2001, 31, 1221–1225 CrossRef CAS.
- E. Aneheim, C. Ekberg and M. R. St. J. Foreman, Solvent Extr. Ion Exch., 2013, 31, 237–252 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and spectra for all compounds. Procedures for solvent extraction measurements. See DOI: 10.1039/c5cc02336j |
| This journal is © The Royal Society of Chemistry 2015 |