Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solubilisation of a 2,2-diphenyl-1-picrylhydrazyl radical in water by β-cyclodextrin to evaluate the radical-scavenging activity of antioxidants in aqueous media†
Ikuo
Nakanishi
*a,
Kei
Ohkubo
bc,
Kohei
Imai
d,
Masato
Kamibayashi
e,
Yasuo
Yoshihashi
f,
Ken-ichiro
Matsumoto
a,
Kiyoshi
Fukuhara
d,
Katsuhide
Terada
f,
Shinobu
Itoh
b,
Toshihiko
Ozawa
g and
Shunichi
Fukuzumi
*bchi
aRadio-Redox-Response Research Team, Advanced Particle Radiation Biology Research Program, Research Center for Charged Particle Therapy, National Institute of Radiological Sciences (NIRS), Inage-ku, Chiba 263-8555, Japan. E-mail: nakanis@nirs.go.jp; Fax: +81-43-255-6819; Tel: +81-43-206-3131
bDepartment of Material and Life Science, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan
cALCA and SENTAN, Japan Science and Technology Agency (JST), Suita, Osaka 565-0871, Japan
dSchool of Pharmacy, Showa University, Shinagawa-ku, Tokyo 142-8555, Japan
ePharmaceutical Manufacturing Chemistry, Kyoto Pharmaceutical University, Kyoto 607-8414, Japan
fDepartment of Pharmaceutics, Faculty of Pharmaceutical Sciences, Toho University, Funabashi, Chiba 274-8510, Japan
gDivision of Oxidative Stress Research, Showa Pharmaceutical University, Machida, Tokyo 194-8543, Japan
hDepartment of Bioinspired Science, Ewha Womans University, Seoul 120-750, Korea
iFaculty of Science and Technology, Meijo University, Shiogamaguchi, Tempaku, Nagoya, Aichi 468-8502, Japan
First published on 8th April 2015
Abstract
A 2,2-diphenyl-1-picrylhydrazyl radical (DPPH˙) was successfully solubilised in water by β-cyclodextrin (β-CD). DPPH˙/β-CD thus obtained was demonstrated to be a powerful tool to evaluate the antioxidative activity of water-soluble antioxidants, such as ascorbate and Trolox, in aqueous buffer solutions.
A relatively stable radical, 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) (Scheme 1), is frequently used as a reactivity model of reactive oxygen species (ROS) to evaluate the radical-scavenging activity of antioxidants.1–6 DPPH˙ shows a characteristic absorption band at around 520 nm, which disappears upon addition of compounds with radical-scavenging activity. However, alcoholic cosolvents, such as methanol and ethanol, are required to use DPPH˙ in aqueous systems due to its little solubility in water.7–9 In such a case, concentrated buffer solutions cannot be used to control the pH of the reaction systems because buffer salts are precipitated in the alcoholic reaction media. Cyclodextrins (CDs) are cyclic oligosaccharides that have a hydrophobic internal cavity and a hydrophilic external surface. Thus, CDs form inclusion complexes with a wide range of hydrophobic molecules and solubilise them in water.10,11 We report herein the solubilisation of DPPH˙ in water using β-cyclodextrin (β-CD: Scheme 1), which consists of 7 glucopyranoside units. The scavenging reaction of β-CD-solubilised DPPH˙ (DPPH˙/β-CD) by water-soluble antioxidants in phosphate buffer solution (0.1 M, pH 7.4) demonstrated that DPPH˙/β-CD would be a powerful tool to evaluate the antioxidative activity in aqueous media without alcoholic cosolvents.
15 mL of boiling water (Milli-Q) or a phosphate buffer solution (0.1 M, pH 7.4) was added to the mixture containing DPPH˙ (0.23 mmol) and β-CD (0.35 mmol), and the suspension was cooled to room temperature. The filtration of the suspension using a membrane filter (pore size: 0.22 μm) gave a deep violet solution. This solution showed an absorption band at 527 nm, which is diagnostic of DPPH˙ (Fig. 1). Thus, DPPH˙ could be solubilised in water by β-CD. A significant red shift of the band due to DPPH˙/β-CD as compared to those of free DPPH˙ in n-hexane (509 nm), MeOH (516 nm), EtOH (517 nm) and acetonitrile (519 nm) suggests that the >N–N˙– moiety of DPPH˙ may exist outside of the β-CD cavity and strongly interact with water. The concentration of DPPH˙ was estimated to be 5.9 × 10−5 M by using the ε value of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 determined for DPPH˙ in a 1
000 M−1 cm−1 determined for DPPH˙ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–buffer solution.9 When β-CD was replaced by α- or γ-CD, which consists of 6 or 8 glucopyranoside units and thus has a smaller or bigger hydrophobic cavity than β-CD, DPPH˙ was not solubilised in water. The 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl radical (DOPPH˙) could not be solubilised in water by β-CD in the same manner, either. Fig. 2a shows an optimised structure of the inclusion complex of DPPH˙ with β-CD calculated by the density functional theory (DFT) (UB3LYP/3-21G:C-PCM solvation model parameterised for water) (see the ESI†). The picryl moiety of DPPH˙ is incorporated into the hydrophobic cavity of β-CD. DOPPH˙ is also incorporated into β-CD as shown in Fig. 2b. The calculated association energy between DPPH˙ and β-CD (−31 kcal mol−1) by DFT is significantly less negative than that between DOPPH˙ and β-CD (−44 kcal mol−1). These theoretical results suggest that DOPPH˙ may not be solubilised by β-CD due to the hydrophobic tert-octyl groups. DPPH˙ solubilised by β-CD in water or the phosphate buffer solution (0.1 M, pH 7.4) is stable at least for several days at room temperature. When a boiling acetate buffer solution (50 mM, pH 4.4) was used instead of the phosphate buffer, DPPH˙ could also be solubilised by β-CD (Fig. 1). On the other hand, a brown solution with absorption bands at 416 and 505 nm was obtained using the boiling borate buffer solution (14 mM, pH 9.1) as shown in Fig. 1. This suggests that DPPH˙ is unstable under basic conditions as reported previously.9 In fact, addition of 0.75 mL of a borate buffer solution (0.1 M, pH 9.1) to DPPH˙/β-CD in water (Milli-Q) (2.3 mL) resulted in a gradual decrease in the absorption band at 527 nm, accompanied by an increase in the band at 412 nm with clear isosbestic points at 252, 304, 346 and 491 nm as shown in Fig. 3. The one-electron reduced DPPH˙ (DPPH−) is reported to have an absorption band at 426 nm in a 1
1 ethanol–buffer solution.9 When β-CD was replaced by α- or γ-CD, which consists of 6 or 8 glucopyranoside units and thus has a smaller or bigger hydrophobic cavity than β-CD, DPPH˙ was not solubilised in water. The 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl radical (DOPPH˙) could not be solubilised in water by β-CD in the same manner, either. Fig. 2a shows an optimised structure of the inclusion complex of DPPH˙ with β-CD calculated by the density functional theory (DFT) (UB3LYP/3-21G:C-PCM solvation model parameterised for water) (see the ESI†). The picryl moiety of DPPH˙ is incorporated into the hydrophobic cavity of β-CD. DOPPH˙ is also incorporated into β-CD as shown in Fig. 2b. The calculated association energy between DPPH˙ and β-CD (−31 kcal mol−1) by DFT is significantly less negative than that between DOPPH˙ and β-CD (−44 kcal mol−1). These theoretical results suggest that DOPPH˙ may not be solubilised by β-CD due to the hydrophobic tert-octyl groups. DPPH˙ solubilised by β-CD in water or the phosphate buffer solution (0.1 M, pH 7.4) is stable at least for several days at room temperature. When a boiling acetate buffer solution (50 mM, pH 4.4) was used instead of the phosphate buffer, DPPH˙ could also be solubilised by β-CD (Fig. 1). On the other hand, a brown solution with absorption bands at 416 and 505 nm was obtained using the boiling borate buffer solution (14 mM, pH 9.1) as shown in Fig. 1. This suggests that DPPH˙ is unstable under basic conditions as reported previously.9 In fact, addition of 0.75 mL of a borate buffer solution (0.1 M, pH 9.1) to DPPH˙/β-CD in water (Milli-Q) (2.3 mL) resulted in a gradual decrease in the absorption band at 527 nm, accompanied by an increase in the band at 412 nm with clear isosbestic points at 252, 304, 346 and 491 nm as shown in Fig. 3. The one-electron reduced DPPH˙ (DPPH−) is reported to have an absorption band at 426 nm in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–buffer solution,9 suggesting that DPPH− may be included in the products. However, the detailed reaction mechanism of DPPH˙ under basic conditions is under investigation and will be reported elsewhere.
1 ethanol–buffer solution,9 suggesting that DPPH− may be included in the products. However, the detailed reaction mechanism of DPPH˙ under basic conditions is under investigation and will be reported elsewhere.
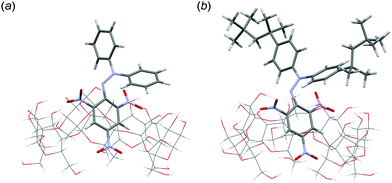 | ||
| Fig. 2 Optimised structures of the inclusion complex of (a) DPPH˙ with β-CD and (b) DOPPH˙ with β-CD calculated by DFT (UB3LYP/3-21G:C-PCM solvation model parameterised for water). | ||
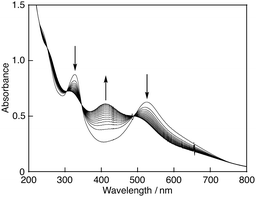 | ||
| Fig. 3 Spectral change observed for DPPH˙/β-CD (5.8 × 10−5 M) in borate buffer (25 mM, pH 9.1) at 298 K. Interval: 20 min. | ||
The EPR spectrum of DPPH˙/β-CD observed in water at room temperature has the same g value (2.0036) and hyperfine coupling constant (7.8 G) as those of DPPH˙ in MeOH (2.0036 and 7.9 G, respectively) (see the Fig. S1, ESI†). A slightly small hyperfine coupling constant (14.6 G) was observed in water as compared to that in MeOH (15.2 G) for the EPR spectra recorded at 77 K (Fig. 4).
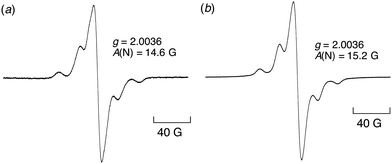 | ||
| Fig. 4 EPR spectra of (a) DPPH˙/β-CD (1.0 × 10−5 M) in distilled water at 77 K and (b) DPPH˙ (1.0 × 10−5 M) in MeOH at 77 K. | ||
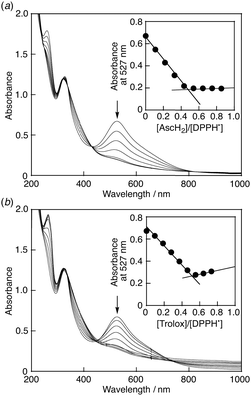
When ascorbic acid (AscH2) was added to the phosphate buffer solution (0.1 M, pH 7.4) of DPPH˙/β-CD, the band at 527 nm disappeared immediately with clear isosbestic points at 320, 338 and 431 nm as shown in Fig. 5a. Since the pKa value of AscH2 is reported to be 4.1,12 AscH2 undergoes deprotonation and exists in its anionic form, AscH−, in phosphate buffer solution (0.1 M, pH 7.4). Thus, this spectral change indicates that AscH− efficiently scavenged DPPH˙ in phosphate buffer [eqn (1)]. When AscH− was replaced by Trolox, a water-soluble analogue of α-tocopherol (vitamin E), a similar spectral change was observed due to the scavenging reaction of DPPH˙ by Trolox [Fig. 5b and eqn (2)].13
| DPPH˙/β-CD + AscH− → DPPH2/β-CD + Asc˙− | (1) |
| DPPH˙/β-CD + Trolox → DPPH2/β-CD + Trolox(−H)˙ | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
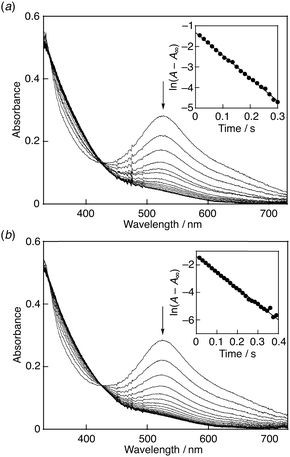
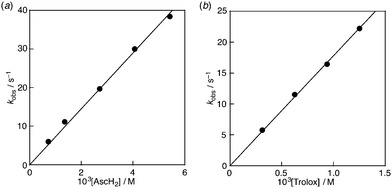
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 The decay of the absorbance at 527 nm monitored by a stopped-flow technique obeyed pseudo-first-order kinetics, when the concentration of AscH2 ([AscH2]) was maintained at more than a 10-fold excess of DPPH˙/β-CD concentration (Fig. 6a). The pseudo-first-order rate constant (kobs) linearly increased with increasing [AscH2] (Fig. 7a). From the slope of the linear plot the second-order rate constant (k) for the scavenging of DPPH˙/β-CD by AscH2 was determined in a phosphate buffer (0.1 M, pH 7.4) to be 7.2 × 103 M−1 s−1. The k value for Trolox was also determined in the same manner to be 1.8 × 104 M−1 s−1 (Fig. 6b and 7b), which is exactly the same as that measured in a 1
1.7 The decay of the absorbance at 527 nm monitored by a stopped-flow technique obeyed pseudo-first-order kinetics, when the concentration of AscH2 ([AscH2]) was maintained at more than a 10-fold excess of DPPH˙/β-CD concentration (Fig. 6a). The pseudo-first-order rate constant (kobs) linearly increased with increasing [AscH2] (Fig. 7a). From the slope of the linear plot the second-order rate constant (k) for the scavenging of DPPH˙/β-CD by AscH2 was determined in a phosphate buffer (0.1 M, pH 7.4) to be 7.2 × 103 M−1 s−1. The k value for Trolox was also determined in the same manner to be 1.8 × 104 M−1 s−1 (Fig. 6b and 7b), which is exactly the same as that measured in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–buffer (pH 7.4) solution.9,14 Thus, β-CD does not inhibit the reaction of DPPH˙ with the antioxidants, while the stability and reactivity of nitroxyl radicals were reported to be significantly changed by the complexation with cyclodextrins.15
1 ethanol–buffer (pH 7.4) solution.9,14 Thus, β-CD does not inhibit the reaction of DPPH˙ with the antioxidants, while the stability and reactivity of nitroxyl radicals were reported to be significantly changed by the complexation with cyclodextrins.15
In summary, β-CD-solubilised DPPH˙ in water has been demonstrated to be a powerful tool to evaluate the antioxidative activity of antioxidants in aqueous media, especially in highly concentrated buffer solutions without precipitation of buffer salts.
This work was partially supported by Grant-in-Aid (No. 26460056 to I.N., 26620154 and 266288037 to K.O.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and ALCA and SENTAN projects from JST.
Notes and references
- M. S. Blois, Nature, 1958, 181, 1199 CrossRef CAS.
- (a) K. U. Ingold and D. A. Pratt, Chem. Rev., 2014, 114, 9022 CrossRef CAS PubMed; (b) G. Litwinienko and K. U. Ingold, Acc. Chem. Res., 2007, 40, 222 CrossRef CAS PubMed.
- (a) E. van Wenum, R. Jurczakowski and G. Litwinienko, J. Org. Chem., 2013, 78, 9102 CrossRef CAS PubMed; (b) M. Musialik, R. Kuzmicz, T. S. Pawłowski and G. Litwinienko, J. Org. Chem., 2009, 74, 2699 CrossRef CAS PubMed; (c) P. Mulder, G. Litwinienko, S. Lin, P. D. MacLean, L. R. Barclay and K. U. Ingold, Chem. Res. Toxicol., 2006, 19, 79 CrossRef CAS PubMed; (d) G. Litwinienko and K. U. Ingold, J. Org. Chem., 2005, 70, 8982 CrossRef CAS PubMed; (e) M. Musialik and G. Litwinienko, Org. Lett., 2005, 7, 495 CrossRef PubMed; (f) G. Litwinienko and K. U. Ingold, J. Org. Chem., 2004, 69, 5888 CrossRef CAS PubMed; (g) G. Litwinienko and K. U. Ingold, J. Org. Chem., 2003, 68, 3433 CrossRef CAS PubMed.
- (a) R. Amorati, S. Menichetti, C. Viglianisi and M. C. Foti, Chem. Commun., 2012, 48, 11904 RSC; (b) M. C. Foti, Int. J. Chem. Kinet., 2012, 44, 524 CrossRef CAS PubMed; (c) V. D. Kancheva, L. Saso, S. E. Angelova, M. C. Foti, A. Slavova-Kasakova, C. Daquino, V. Enchev, O. Firuzi and J. Nechev, Biochemie, 2012, 94, 403 CrossRef CAS PubMed; (d) M. C. Foti, C. Daquino, G. A. DiLabio and K. U. Ingold, Org. Lett., 2011, 13, 4826 CrossRef CAS PubMed; (e) M. C. Foti, R. Amorati, G. F. Pedulli, C. Daquino, D. A. Pratt and K. U. Ingold, J. Org. Chem., 2011, 75, 4434 CrossRef PubMed; (f) M. C. Foti, C. Daquino, I. D. Mackie, G. A. DiLabio and K. U. Ingold, J. Org. Chem., 2008, 73, 9270 CrossRef CAS PubMed; (g) M. C. Foti and C. Daquino, Chem. Commun., 2006, 3252 RSC; (h) M. C. Foti, C. Daquino and C. Geraci, J. Org. Chem., 2004, 69, 2309 CrossRef CAS PubMed; (i) M. C. Foti, L. R. Barlay and K. U. Ingold, J. Am. Chem. Soc., 2002, 124, 12881 CrossRef CAS PubMed.
- (a) T. Waki, S. Kobayashi, K. Matsumoto, T. Ozawa, T. Kamada and I. Nakanishi, Chem. Commun., 2013, 49, 9842 RSC; (b) H. P. Indo, I. Nakanishi, K. Ohkubo, H. Yen, M. Nyui, S. Manda, K. Matsumoto, K. Fukuhara, K. Anzai, N. Ikota, H. Matsui, Y. Minamiyama, A. Nakajima, H. Ichikawa, S. Fukuzumi, T. Ozawa, C. Mukai and H. J. Majima, RSC Adv., 2013, 3, 4535 RSC; (c) T. Kawashima, S. Manda, Y. Uto, K. Ohkubo, H. Hori, K. Matsumoto, K. Fukuhara, N. Ikota, S. Fukuzumi, T. Ozawa, K. Anzai and I. Nakanishi, Bull. Chem. Soc. Jpn., 2012, 85, 877 CrossRef CAS; (d) T. Waki, I. Nakanishi, K. Matsumoto, J. Kitajima, T. Chikuma and S. Kobayashi, Chem. Pharm. Bull., 2012, 60, 37 CrossRef CAS; (e) S. Kobayashi, T. Waki, I. Nakanishi, K. Matsumoto and K. Anzai, Chem. Pharm. Bull., 2010, 58, 1442 CrossRef CAS; (f) I. Nakanishi, T. Kawashima, K. Ohkubo, H. Kanazawa, K. Inami, M. Mochizuki, K. Fukuhara, H. Okuda, T. Ozawa, S. Itoh, S. Fukuzumi and N. Ikota, Org. Biomol. Chem., 2005, 3, 626 RSC.
- S. B. Kedare and R. P. Singh, J. Food Sci. Technol., 2011, 48, 412 CrossRef CAS PubMed and references cited therein.
- M. B. Arnao, Trends Food Sci. Technol., 2000, 11, 419 CrossRef CAS.
- D.-W. Li, F.-F. Tian, Y.-S. Ge, X.-L. Ding, J.-H. Li, Z.-Q. Xu, M.-F. Zhang, X.-L. Han, R. Li, F.-L. Jiang and Y. Liu, Chem. Commun., 2011, 47, 10713 RSC.
- O. Friaa and D. Brault, Org. Biomol. Chem., 2006, 4, 2417 CAS.
- G. Crini, Chem. Rev., 2014, 114, 10940 CrossRef CAS PubMed and references cited therein.
- I. Nakanishi, S. Fukuzumi, T. Konishi, K. Ohkubo, M. Fujitsuka, O. Ito and N. Miyata, J. Phys. Chem. B, 2002, 106, 2372 CrossRef CAS.
- (a) C. Creutz, Inorg. Chem., 1981, 20, 4449 CrossRef CAS; (b) N. H. Williams and J. K. Yandell, Aust. J. Chem., 1982, 35, 1133 CrossRef CAS.
- Clear isosbestic points such as those in Fig. 3a were not observed due to relatively poor solubility of Trolox in phosphate buffer as compared to AscH2.
- For 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water mixtures, the k values were reported to be 8.6 × 103 and 7.0 × 103 M−1 s−1, respectively. See ref. 9.
1 ethanol–water mixtures, the k values were reported to be 8.6 × 103 and 7.0 × 103 M−1 s−1, respectively. See ref. 9. - (a) M. Spulber and S. Schlick, J. Phys. Chem. A, 2010, 114, 6217 CrossRef CAS PubMed; (b) D. Bardelang, L. Charles, J.-P. Finet, L. Jicsinszky, H. Karoui, S. R. A. Marque, V. Monnier, A. Rockenbauer, R. Rosas and P. Tordo, Chem.– Eur. J., 2007, 13, 9344 CrossRef CAS PubMed; (c) H. Karoui and P. Tordo, Tetrahedron Lett., 2004, 45, 1043 CrossRef CAS PubMed; (d) H. Karoui, A. Rockenbauer, S. Pietri and P. Tordo, Chem. Commun., 2002, 3030 RSC; (e) C. Ebel, K. U. Ingold, J. Michon and A. Rassat, Tetrahedron Lett., 1985, 26, 741 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and EPR spectra at room temperature (Fig. S1). See DOI: 10.1039/c5cc02236c |
| This journal is © The Royal Society of Chemistry 2015 |

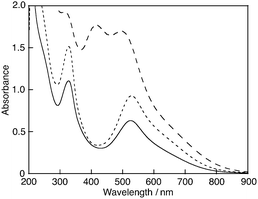
![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) and borate buffer (14 mM, pH 9.1) (– – –).
) and borate buffer (14 mM, pH 9.1) (– – –).