Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible light-induced singlet oxygen-mediated intracellular disassembly of polymeric micelles co-loaded with a photosensitizer and an anticancer drug for enhanced photodynamic therapy†
Gurusamy
Saravanakumar
,
Junseok
Lee
,
Jihoon
Kim
and
Won Jong
Kim
*
Center for Self-assembly and Complexity, Institute of Basic Science (IBS), and Department of Chemistry, Pohang University of Science and Technology, Pohang 790-784, Republic of Korea. E-mail: wjkim@postech.ac.kr; Fax: +82-279-3399; Tel: +82-57-279-2104
First published on 12th May 2015
Abstract
Herein, we report a biocompatible amphiphilic block copolymer micelle bearing a singlet oxygen-sensitive vinyldithioether cleavable linker at the core–shell junction, which undergoes singlet oxygen-mediated photocleavage in the presence of visible light. The micelle facilitates the light-responsive release of singlet oxygen and an anticancer drug for enhanced photodynamic therapy.
Photodynamic therapy (PDT) is emerging as a promising treatment modality for several cancers and other non-malignant diseases owing to its unique advantages such as non-invasiveness and less systemic toxicity.1 This modality works by administering a photosensitizer (PS) and then activating it with a light of specific wavelength to generate reactive oxygen species, primarily singlet oxygen (1O2), which induce apoptosis or necrosis of the tumor cells. Despite its significant advantages, however, the clinical potential of PDT is limited by factors such as poor aqueous solubility of PSs and nonspecific accumulation of PSs at the normal tissues. To surmount these issues, several nanocarrier platforms such as liposomes,2 polymeric micelles,3 dendrimers,4 and self-assembled polymeric nanoparticles5 have been investigated. Another important concern of PDT is the depletion of ambient tumor oxygen through rapid and continuous photochemical conversion of 3O2 to 1O2 at high fluence rates, which can cause acute local hypoxic conditions and disrupt the photodynamic process, thereby reducing the treatment effectiveness.6
Integration of PDT with other treatment modalities such as photothermal therapy7 or chemotherapy8 holds great promise to circumvent the above critical limitation, because such a combination therapy produces additive or synergistic therapeutic efficacy by targeting different cellular or molecular pathways. It is particularly gratifying to note that the results from recent preclinical studies have also indicated that combining PDT with chemotherapy can improve anticancer activity by overcoming anticancer drug resistance9 which is a major impediment to the success of chemotherapy. Considering highly liphophilic and molecularly diverse characteristics of both PSs and anticancer drugs, however, the development of smart and efficient carriers for the co-delivery of PSs and anticancer drugs is crucial to realize the full potential of the combination therapy.
For such co-delivery, we envisioned that polymeric micelles integrated with photo-responsive moieties could be a potential carrier. Firstly, its core–shell structures could readily encapsulate multiple poorly water-soluble active agents into the hydrophobic core with high loading capacity. Secondly, without any additional trigger mechanism, the co-loaded anticancer drugs can be effectively released in the tumor cell to exert their therapeutic effects because light is an integral part of the PDT process. Although light-responsive micellar systems have been developed by incorporating photochromic moieties such as azobenzene,10 2-diazo-1,2-napthoquinone,11O-nitrobenzyl12 or coumarin13 into their structure, unfortunately, most of them respond only to the harmful UV light or multi-photon excitation that requires enormously high laser power. Moreover, these systems are mostly based on non-degradable polymeric compositions with limited biocompatibility. Thus, there is an increasing need to develop safe, low toxic and more efficient carrier systems that could respond to the biomedically benign visible or near-infra-red light. It should also be emphasized that, to date, no significant effort has been made to the development of rationally designed light-responsive carriers for dual-drug delivery.
In this study, we have designed and explored a novel biocompatible visible-light responsive amphiphilic poly(ethylene glycol)-block-poly(caprolactone) copolymer (1O2-PEG-b-PCL) capable of forming micelles by incorporating an 1O2-sensitive vinyldithioether linker between the hydrophilic PEG and the hydrophobic and biodegradable PCL block. The double bond of the vinyldithioether linker can readily react with 1O2 to form a dioxetane intermediate, which is unstable and spontaneously cleaved into fragments. Therefore, unlike other conventional photo-responsive micelles that demand direct absorption of UV light, our micellar system exploits the 1O2 generated by visible light during PDT as a trigger to disassemble the nanostructure and release a co-loaded drug in the tumor cell for effective therapy (Scheme 1). Furthermore, the cleaved fragments do not produce any toxic species like other photodegradable moieties. The potential of the micelle as a dual-drug carrier for enhanced photodynamic therapy was investigated by physical co-encapsulation of a PS, chlorine e6 (Ce6), and a hydrophobic anticancer drug, doxorubicin (DOX).
The synthetic strategy of a biocompatible 1O2-PEG-b-PCL copolymer involves three steps (as illustrated in Fig. S1, ESI†). First, a 1O2-sensitive vinyldithioether linker was synthesized following a reported method,14 and its 1H-NMR spectrum (Fig. S2, ESI†) was in good agreement with the literature. Second, a PEG macroinitiator (1O2-PEG-OH) bearing an 1O2-sensitive linker was synthesized by coupling a carboxylated PEG to the excess of as-synthesized linker via Steglich esterification. The successful conjugation was confirmed by the characteristic cis-alkene peak (∼SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHS∼) at δ 6.12 ppm from the linker and the PEG peaks (CH3–O– at δ 3.36 ppm, and –CH2CH2– at δ 3.62 ppm) in the 1H-NMR spectrum of 1O2-PEG-OH (Fig. S3a, ESI†). Third, the 1O2-PEG-b-PCL copolymer was synthesized by ring-opening polymerization of the ε-caprolactone (ε-CL) monomer using the 1O2-PEG-OH as a macroinitiator in the presence of Sn(Oct)2 as a catalyst. The chemical structure of the block copolymer was confirmed by 1H-NMR spectroscopy (Fig. S3b, ESI†), in which all the resonance peaks corresponding to the PEG and PCL segments were observed. Further, the composition of the copolymer was estimated by comparing the integration ratios of the peaks at 3.35 ppm from the methyl protons (CH3–) of PEG and the peak at 4.03 ppm from the methylene protons (–CH2–O–) of PCL segments. GPC analysis of the block copolymer showed a unimodal molecular weight distribution (Fig. S4, ESI†). For comparison, a control C-PEG-b-PCL copolymer of similar composition without the linker was also synthesized. The compositions of these block copolymers are summarized in Table S1 (ESI†).
CHS∼) at δ 6.12 ppm from the linker and the PEG peaks (CH3–O– at δ 3.36 ppm, and –CH2CH2– at δ 3.62 ppm) in the 1H-NMR spectrum of 1O2-PEG-OH (Fig. S3a, ESI†). Third, the 1O2-PEG-b-PCL copolymer was synthesized by ring-opening polymerization of the ε-caprolactone (ε-CL) monomer using the 1O2-PEG-OH as a macroinitiator in the presence of Sn(Oct)2 as a catalyst. The chemical structure of the block copolymer was confirmed by 1H-NMR spectroscopy (Fig. S3b, ESI†), in which all the resonance peaks corresponding to the PEG and PCL segments were observed. Further, the composition of the copolymer was estimated by comparing the integration ratios of the peaks at 3.35 ppm from the methyl protons (CH3–) of PEG and the peak at 4.03 ppm from the methylene protons (–CH2–O–) of PCL segments. GPC analysis of the block copolymer showed a unimodal molecular weight distribution (Fig. S4, ESI†). For comparison, a control C-PEG-b-PCL copolymer of similar composition without the linker was also synthesized. The compositions of these block copolymers are summarized in Table S1 (ESI†).
Owing to its amphiphilicity, the 1O2-PEG-b-PCL copolymer can form a self-assembled micellar structure (1O2-PM) under aqueous conditions. The dynamic light scattering measurements of micelles showed unimodal size distribution with an average particle size of 40 ± 3.1 nm and the TEM image indicated that they have a spherical morphology (Fig. S5, ESI†). Since the 1O2-PM is incorporated with a 1O2-sensitive linker, first we investigated whether the 1O2-PEG-b-PCL copolymer can undergo visible-light induced 1O2-mediated photocleavage. To investigate the photocleavage, the 1H-HMR spectra of the block copolymer solution containing 5 wt% Ce6 without and with exposure to laser irradiation (30 mW cm−2, 3 h) were recorded. As shown in Fig. S6a (ESI†), the characteristic cis-alkene peak at δ 6.1 ppm of the linker completely disappeared, whereas all the characteristic peaks of the polymers were observed without laser irradiation. This result was further supported by the GPC analysis (Fig. S6b, ESI†), which showed the appearance of low molecular weight traces corresponding to the 1O2-PEG-macroinitiator for the laser irradiated polymer. The above results clearly indicate that the block copolymer fragments into their two constituent blocks under laser irradiation.
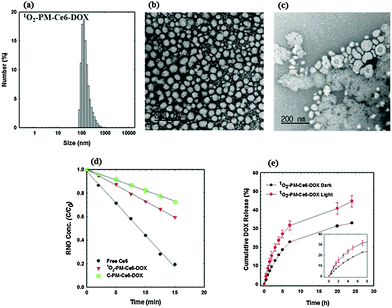
After confirming the 1O2-mediated photocleavage of the 1O2-PEG-b-PCL copolymer, its potential as a carrier for co-delivery of a PS and an anticancer drug was investigated by encapsulating Ce6 and DOX via the dialysis method. For comparison, micelles loaded with only Ce6 were also prepared using the same method. The drug loading contents and loading efficiencies of these micelles are summarized in Table S2 (ESI†). The size of Ce6 and DOX co-loaded micelles (1O2-PM-Ce6-DOX, Fig. 1a) was higher than Ce6-loaded micelles (1O2-PM-Ce6, Fig. S7a, ESI†), indicating that the co-loading of Ce6 and DOX in the hydrophobic core increases the volume of micelles. The TEM images showed spherical morphologies for both 1O2-PM-Ce6-DOX (Fig. 1b) and 1O2-PM-Ce6 (Fig. S7b, ESI†), although the size obtained by TEM is smaller than the DLS measurement due to the non-hydrated state. The size distributions of the micelles prepared using the block polymer without the 1O2-linker are shown in Fig. S7c (ESI†). To investigate whether 1O2-PM-Ce6-DOX micelles can be disassembled by visible light, the TEM image of the micelles was recorded after exposing them to laser light irradiation (660 nm, 50 mW cm−2, 3 h). As expected, compared to the spherical micelles before irradiation, highly degraded irregular aggregated micelles were observed after laser irradiation (Fig. 1c). This clearly indicates that light-induced 1O2-mediated photocleavage of the linker could disrupt the micellar structures by disturbing the hydrophilic–liphophilic balance. The UV-vis spectrum of 1O2-PM-Ce6 showed a similar characteristic Soret band and Q-band absorption of free Ce6, indicating that the Ce6 in micelles existed in non-aggregated form (Fig. S8, ESI†). The monomeric form is very important to ensure the photo-physical characteristic of Ce6 for efficient 1O2 generation. The 1O2-generation efficiency of 1O2-PM-Ce6-DOX was evaluated using the N,N-dimethyl-4-nitrosoaniline (RNO)-histidine colorimetric assay.15 In this assay, 1O2 generated from the photoexcited sensitizer reacts with the imidazole moiety and forms a transient complex that bleaches the RNO molecules. Thus, the amount of 1O2 can be directly correlated with a decrease in RNO absorbance in the UV-vis spectrum. Fig. 1d shows the RNO concentration as a function of time for free Ce6, 1O2-PM-Ce6-DOX and C-PM-Ce6-DOX. Upon irradiation, free Ce6 showed a sharp decrease in RNO concentration, indicating rapid 1O2 generation. Although micelles showed a slow decrease in absorbance compared to the free Ce6, 1O2-PM-Ce6-DOX showed a slightly fast decrease in RNO concentration as compared to C-PM-Ce6-DOX. This increase in 1O2 generation efficiency of 1O2-PM-Ce6-DOX could be due to the disassembly of micelles under laser irradiation, which releases the Ce6 molecules in monomeric form. Next, the drug release behaviour of the 1O2-PM-Ce6-DOX was investigated in PBS (pH 7.4) with and without light. The cumulative release percentage of DOX loaded in micelles versus time is shown in Fig. 1e. The DOX release rates were accelerated in the presence of light irradiation compared to the dark conditions. The fast DOX release from the micelles was most likely due to the degradation micelles by the photocleavage of the vinyldithioether bonds. These results suggest that micelles incorporated with a 1O2-sensitive linker can effectively release co-loaded drugs under photodynamic conditions at the target cells, which may improve the overall therapeutic efficacy.
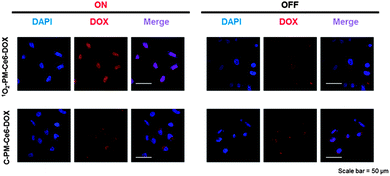
As designed, the light-responsive release of DOX from 1O2-PM-Ce6-DOX was deemed to exhibit the spatio-temporal controlled synergistic anticancer effects. Therefore, the therapeutic potential of 1O2-PM-Ce6-DOX was evaluated in vitro. Irrespective of light on/off, the 1O2-PM and C-PM micelles demonstrated insignificant cytotoxicity due to the biocompatibility of PEG and PCL (Fig. S9, ESI†). In addition, no significant toxicity was observed with 1O2-PM-Ce6 and C-PM-Ce6 micelles under dark conditions (Fig. 2a). However, 1O2-PM-Ce6 and C-PM-Ce6 micelles showed slight cytotoxicity under light irradiation, which was ascribed to the cytotoxic effects of 1O2 generated from light-activated Ce6 (Fig. 2a). In the cases of Ce6 and DOX loaded micelles under dark conditions, cell viability was retained above 95% for micellar concentrations of up to 5 μg mL−1. The insignificant toxicity of DOX-releasing micelles was due to the lower concentration of DOX compared with general DOX doses16 for the in vitro test. Although each of 1O2 and DOX released from the micelles could not exert the effective cytotoxic effects on the cancer cells, the combinatorial use of them showed the significant anticancer efficiency. Especially, under light irradiation, the 1O2-PM-Ce6-DOX micelle showed the most efficient therapeutic effects compared to the C-PM-Ce6-DOX micelle (Fig. 2b). To demonstrate the potential of the micelles for intracellular delivery, cellular uptake and intracellular release behaviour of DOX from the micelles were evaluated using confocal laser scan microscopy. As shown in Fig. 3, the strongest fluorescence in the nucleus was observed in the cells incubated with 1O2-PM-Ce6-DOX micelles exposed to light compared to the dark conditions and control micelles, which could be attributed to the enhanced release of DOX by 1O2-responsive destruction of the micelle. Therefore, it can be concluded that the 1O2-PM-Ce6-DOX micelle showed the potential not only for reducing the unintended cytotoxic effects on the normal tissues during blood circulation, but also for exhibiting the effective anticancer effects only on tumours.
 | ||
| Fig. 2 Laser-induced cellular photo-toxicity of (a) 1O2-PM-Ce6 and C-PM-Ce6 micelles, and (b) 1O2-PM-Ce6-DOX and C-PM-Ce6-DOX micelles in PC3 cell lines. | ||
In summary, visible light-triggered intracellular co-delivery of an anticancer drug and a PS using biocompatible polymeric micelles bearing vinyldithioether as a 1O2 cleavable linker is realized for more effective photodynamic therapy. Given the high biocompatibility and ability to release drugs under photodynamic therapy conditions, the micellar system developed in this study could be a promising carrier for co-delivery of hydrophobic therapeutic drugs and PSs.
This work was supported by the Research Center Program of IBS (Institute for Basic Science) in Korea (CA1203-02).
Notes and references
- (a) D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380 CrossRef CAS PubMed; (b) T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, J. Natl. Cancer Inst., 1998, 90, 889 CrossRef CAS PubMed; (c) I. J. Macdonald and T. J. Dougherty, J. Porphyrins Phthalocyanines, 2001, 5, 105 CrossRef CAS PubMed.
- A. S. L. Derycke and P. A. M. de Witte, Adv. Drug Delivery Rev., 2004, 56, 17 CrossRef CAS PubMed.
- (a) C. F. van Nostrum, Adv. Drug Delivery Rev., 2004, 56, 9 CrossRef CAS PubMed; (b) B. Li, E. H. Moriyama, F. Li, M. T. Jarvi, C. Allen and B. C. Wilson, Photochem. Photobiol., 2007, 83, 1505 CrossRef CAS PubMed; (c) H. Ding, B. D. Sumer, C. W. Kessinger, Y. Dong, G. Huang, D. A. Boothman and J. Gao, J. Controlled Release, 2011, 151, 271 CrossRef CAS PubMed.
- N. Nishiyama, Y. Morimoto, W.-D. Jang and K. Kataoka, Adv. Drug Delivery Rev., 2009, 61, 327 CrossRef CAS PubMed.
- S. J. Lee, K. Park, Y.-K. Oh, S.-H. Kwon, S. Her, I.-S. Kim, K. Choi, S. J. Lee, H. Kim, S. G. Lee, K. Kim and I. C. Kwon, Biomaterials, 2009, 30, 2929 CrossRef CAS PubMed.
- B. W. Henderson, T. M. Busch, L. A. Vaughan, N. P. Frawley, D. Babich, T. A. Sosa, J. D. Zollo, A. S. Dee, M. T. Cooper, D. A. Bellnier, W. R. Greco and A. R. Oseroff, Cancer Res., 2000, 60, 525 CAS.
- (a) B. Jang, J.-Y. Park, C.-H. Tung, I.-H. Kim and Y. Choi, ACS Nano, 2011, 5, 1086 CrossRef CAS PubMed; (b) M. Zhang, T. Murakami, K. Ajima, K. Tsuchida, A. S. D. Sandanayaka, O. Ito, S. Iijima and M. Yudasaka, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14773 CrossRef CAS PubMed.
- J. W. Snyder, W. R. Greco, D. A. Bellnier, L. Vaughan and B. W. Henderson, Cancer Res., 2003, 63, 8126 CAS.
- A. Khdair, C. Di, Y. Patil, L. Ma, Q. P. Dou, M. P. V. Shekhar and J. Panyam, J. Controlled Release, 2010, 141, 137 CrossRef CAS PubMed.
- Q. Yan, Y. Xin, R. Zhou, Y. Yin and J. Yuan, Chem. Commun., 2011, 47, 9594 RSC.
- (a) L. Sun, X. Ma, C.-M. Dong, B. Zhu and X. Zhu, Biomacromolecules, 2012, 13, 3581 CrossRef CAS PubMed; (b) G.-Y. Liu, C.-J. Chen, D.-D. Li, S.-S. Wang and J. Ji, J. Mater. Chem., 2012, 22, 16865 RSC.
- G. Liu and C.-M. Dong, Biomacromolecules, 2012, 13, 1573 CrossRef CAS PubMed.
- J. Babin, M. Pelletier, M. Lepage, J.-F. Allard, D. Morris and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 3329 CrossRef CAS PubMed.
- S. D. P. Baugh, Z. Yang, D. K. Leung, D. M. Wilson and R. Breslow, J. Am. Chem. Soc., 2001, 123, 12488 CrossRef CAS PubMed.
- I. Kraljić and S. E. Mohsni, Photochem. Photobiol., 1978, 28, 577 CrossRef PubMed.
- J. Lee, C. Jeong and W. J. Kim, J. Mater. Chem. B, 2014, 2, 8338 RSC.
Footnote |
| † Electronic supplementary information (ESI): Experimental details, supplementary figures and tables. See DOI: 10.1039/c5cc01937k |
| This journal is © The Royal Society of Chemistry 2015 |