Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Helical mesoporous silica as an inorganic heterogeneous chiral trigger for asymmetric autocatalysis with amplification of enantiomeric excess†
Tsuneomi
Kawasaki‡
ab,
Yuko
Araki
a,
Kunihiko
Hatase
a,
Kenta
Suzuki
a,
Arimasa
Matsumoto
a,
Toshiyuki
Yokoi
c,
Yoshihiro
Kubota
d,
Takashi
Tatsumi
*c and
Kenso
Soai
*ab
aDepartment of Applied Chemistry, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo, 162-8601, Japan. E-mail: soai@rs.kagu.tus.ac.jp
bResearch Center for Chirality, Research Institute for Science and Technology (RIST), Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo, 162-8601, Japan
cChemical Resources Laboratory, Tokyo Institute of Technology, Nagatsuta, Midori-ku, Yokohama 226-8503, Japan. E-mail: ttatsumi@cat.res.titech.ac.jp
dDivision of Materials Science and Chemical Engineering, Yokohama National University, 79-5, Tokiwadai, Hodogaya-ku, Yokohama, 240-8501, Japan
First published on 16th March 2015
Abstract
Mesoporous silica has been used as a heterogeneous support for catalysts; however, asymmetric induction by the helicity of inorganic mesoporous silica itself has not yet been achieved. P- and M-helical mesoporous silica was found to act as a chiral inorganic trigger for asymmetric autocatalysis to afford (S) and (R)-pyrimidyl alkanol with >99.5% ee, respectively.
Catalytic enantioselective synthesis has attracted much attention in the fields of materials science and pharmaceuticals, because it is a useful method for synthesizing a large amount of a chiral compound from an initial small amount of chiral compound.1 From an environmental and economic point of view, asymmetric synthesis using heterogeneous inorganic materials is an emerging area, because these catalysts can be separated from the reaction mixture easily and their thermal and mechanical stability allows for the recovery and reuse of the catalyst.2,3
On the other hand, research into well-ordered mesoporous materials has become a “hot area” in materials science4 because of their versatile applications in areas such as catalysis, adsorption, and drug delivery systems.5 Their extremely large surface area, uniform pore size, and well-defined pore shape enable them to act as selective catalysts. There are some examples of chiral catalyst supported on achiral mesoporous silica.6 However, to the best of our knowledge, there has been no report on asymmetric reaction induced by the chirality of inorganic helical mesoporous silica itself. Therefore, the use of mesoporous materials with a chiral inorganic framework presents a challenge in the field of asymmetric catalysis.
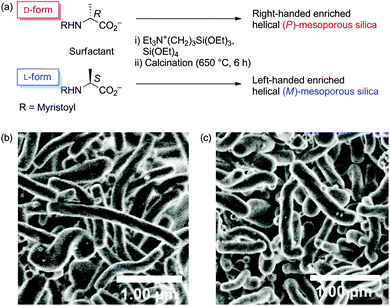
One of us synthesized chiral mesoporous silica using N-myristoyl alanine as a chiral anionic surfactant, which has a twisted hexagonal rod-like morphology, with dimensions of diameter = 130–180 nm and length = 1–6 μm.7 Right-handed enriched (P)-helical mesoporous silica has been produced from a surfactant derived from D-alanine. In contrast, the L-isomer afforded left-handed enriched (M)-helical mesoporous silica (Fig. 1). To the best of our knowledge, there have been no reports on mesoporous silica mediated asymmetric synthesis in which the chiral inorganic structure induces the enantioselectivity.
Meanwhile, we have continued to study asymmetric autocatalysis with the amplification of enantiomeric excess (ee)8 where a chiral product acts as a chiral catalyst for its own production.9 Chiral compounds can act as the source of chirality in the addition reaction of diisopropylzinc (i-Pr2Zn) to aldehydes to give highly enantioenriched alkanols in combination with asymmetric autocatalysis.10–14
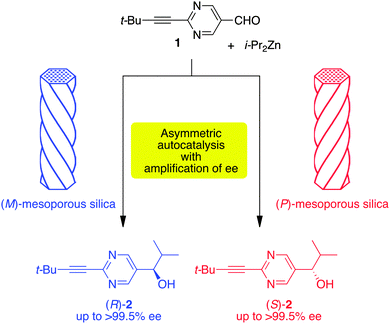
Here, we report that chiral mesoporous silica acts as a heterogeneous chiral catalyst in the enantioselective addition of iPr2Zn to pyrimidine-5-carbaldehyde 1 to afford, in combination with asymmetric autocatalysis, 5-pyrimidyl alkanol 2 with a high ee (Scheme 1).
The results of asymmetric autocatalysis in the presence of helical mesoporous silica7 are summarized in Table 1. In the presence of right-handed enriched helical (P)-mesoporous silica (Sample A) with approximately 52% ee, (S)-5-pyrimidyl alkanol with a 97% ee was obtained in a 92% isolated yield from the reaction of pyrimidine-5-carbaldehyde 1 and i-Pr2Zn (entry 1). When Sample A with a (P)-helicity was used as the chiral promoter, the same stereochemical outcome was observed, i.e., 5-pyrimidyl alkanol 2 with S chirality was produced in a reproducible manner (entries 2 and 3). In sharp contrast, left-handed enriched (M)-mesoporous silica Sample B promoted the formation of (R)-pyrimidyl alkanol 2 with an 80% ee in an 85% yield (entry 4). The stereochemical correlation using Sample B was reproducible and afforded 5-pyrimidyl alkanol 2 possessing R-chirality (entries 5 and 6). For entries 7 and 8, another sample of helical mesoporous silica, Sample C, was subjected to asymmetric autocatalysis. The correlation between the enrichment of the (P)-helicity of the silica and the (S)-configuration of alkanol 2 was reproduced well (entries 7 and 8). The formation of (R)-2 was reproducible when chiral (M)-enriched mesoporous silica Samples D and E were utilized as the chiral promoters of asymmetric autocatalysis (entries 9–11). It should be noted that near enantiopure products (S)- and (R)-2 with >99.5% ee could be obtained by consecutive asymmetric autocatalysis (entries 8 and 10, respectively).8b
| Entry | Mesoporous silicab | 5-Pyrimidyl alkanol 2 | ||||
|---|---|---|---|---|---|---|
| Lot no. | Chirality | eec (%) | Yieldd (%) | eee (%) | Config. | |
a The molar ratio used was chiral mesoporous silica (SiO2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrimidine-5-carbaldehyde 1 pyrimidine-5-carbaldehyde 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-Pr2Zn = 0.09 i-Pr2Zn = 0.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.53 0.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.18.
b Helical mesoporous silica was prepared according to the reported method.7
c The ee of the chiral mesoporous silica was checked using SEM images from each sample (see the ESI).
d Isolated yield.
e The ee was determined using HPLC employing a chiral stationary phase.
f The typical experimental procedure used is as follows: to a mixture of right-handed enriched (P)-mesoporous silica (30 mg) with ca. 52% ee and pyrimidine-5-carbaldehyde 1 (9.4 mg, 0.05 mmol) was added a solution of i-Pr2Zn (0.15 mmol, 0.15 mL of 1 M toluene solution) at 0 °C, and the mixture was then stirred for 4 h. After the addition of toluene (4.75 mL), i-Pr2Zn (0.4 mmol, 0.4 mL of a 1 M toluene solution) was added over a period of 30 min at 0 °C. After stirring the mixture for 15 min, a solution of 1 (37.6 mg, 0.2 mmol) in toluene (1.5 mL) was added over a period of 1.5 h. After the mixture was left stirring overnight, toluene (14.4 mL) and a solution of i-Pr2Zn (1.6 mmol, 1.6 mL of 1 M toluene solution) were added successively, and the resulting mixture was stirred for a further 20 min. A solution of 1 (150.6 mg, 0.8 mmol) in toluene (4.0 mL) was then added over a period of 1 h at 0 °C. After stirring for a further 2 h, the reaction was quenched using 1 M hydrochloric acid (5 mL) and neutralized with a saturated sodium hydrogen carbonate solution (15 mL). The mixture was then filtered using Celite, and the filtrate was extracted three times using ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate and evaporated in vacuo. Purification of the residue using silica gel column chromatography (hexane/ethyl acetate = 3/1, v/v) gave the (S)-5-pyrimidyl alkanol 2 (225 mg, 0.968 mmol) with a 97% ee in a yield of 92%. The ee was determined using HPLC, employing a chiral stationary phase (Daicel Chiralcel OD–H, 4.6 × 250 mm, eluent: 2-propanol/hexane = 5/95 (v/v), flow rate: 1.0 mL min−1, 254 nm UV detector, retention time: 10.1 min for (S)-2, 15.1 min for (R)-2).
g After carrying out a typical experimental procedure, additional rounds of consecutive asymmetric autocatalysis were performed.8b 1.18.
b Helical mesoporous silica was prepared according to the reported method.7
c The ee of the chiral mesoporous silica was checked using SEM images from each sample (see the ESI).
d Isolated yield.
e The ee was determined using HPLC employing a chiral stationary phase.
f The typical experimental procedure used is as follows: to a mixture of right-handed enriched (P)-mesoporous silica (30 mg) with ca. 52% ee and pyrimidine-5-carbaldehyde 1 (9.4 mg, 0.05 mmol) was added a solution of i-Pr2Zn (0.15 mmol, 0.15 mL of 1 M toluene solution) at 0 °C, and the mixture was then stirred for 4 h. After the addition of toluene (4.75 mL), i-Pr2Zn (0.4 mmol, 0.4 mL of a 1 M toluene solution) was added over a period of 30 min at 0 °C. After stirring the mixture for 15 min, a solution of 1 (37.6 mg, 0.2 mmol) in toluene (1.5 mL) was added over a period of 1.5 h. After the mixture was left stirring overnight, toluene (14.4 mL) and a solution of i-Pr2Zn (1.6 mmol, 1.6 mL of 1 M toluene solution) were added successively, and the resulting mixture was stirred for a further 20 min. A solution of 1 (150.6 mg, 0.8 mmol) in toluene (4.0 mL) was then added over a period of 1 h at 0 °C. After stirring for a further 2 h, the reaction was quenched using 1 M hydrochloric acid (5 mL) and neutralized with a saturated sodium hydrogen carbonate solution (15 mL). The mixture was then filtered using Celite, and the filtrate was extracted three times using ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate and evaporated in vacuo. Purification of the residue using silica gel column chromatography (hexane/ethyl acetate = 3/1, v/v) gave the (S)-5-pyrimidyl alkanol 2 (225 mg, 0.968 mmol) with a 97% ee in a yield of 92%. The ee was determined using HPLC, employing a chiral stationary phase (Daicel Chiralcel OD–H, 4.6 × 250 mm, eluent: 2-propanol/hexane = 5/95 (v/v), flow rate: 1.0 mL min−1, 254 nm UV detector, retention time: 10.1 min for (S)-2, 15.1 min for (R)-2).
g After carrying out a typical experimental procedure, additional rounds of consecutive asymmetric autocatalysis were performed.8b
|
||||||
| 1f | A | P | 52 | 92 | 97 | S |
| 2 | A | P | 52 | 92 | 92 | S |
| 3 | A | P | 52 | 91 | 86 | S |
| 4 | B | M | 19 | 85 | 80 | R |
| 5 | B | M | 19 | 83 | 82 | R |
| 6 | B | M | 19 | 78 | 97 | R |
| 7 | C | P | 39 | 87 | 92 | S |
| 8 | C | P | 39 | 85 | 96g (>99.5) | S |
| 9 | D | M | 26 | 89 | 86 | R |
| 10 | D | M | 26 | 93 | 93g (>99.5) | R |
| 11 | E | M | 22 | 91 | 94 | R |
Asymmetric autocatalysis is a very powerful reaction for detecting low levels of chirality (crypto chirality) in a substance15 and, therefore, to ensure that asymmetric induction that occurred was due to the helical structure of the mesoporous silica, thermal analysis (TG–DTA) was performed. As shown in Fig. 2, the organic part completely disappeared around 600 °C. Since the chiral mesoporous silica was prepared by calcining the composite precursor at 650 °C for 6 h, only the chiral inorganic framework would remain to act as the chiral source for the enantioselective reaction.16
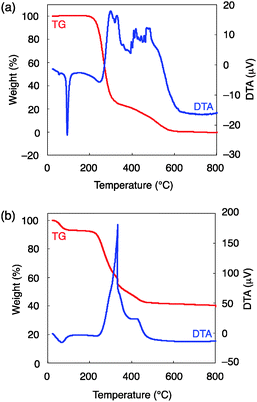 | ||
| Fig. 2 Thermal analysis (TG–DTA) of (a) N-myristoyl-L-alanine (5.4 mg, 10 °C min−1, air, 30 mL min−1) and (b) the surfactant–silica composite (10.1 mg, 10 °C min−1, air, 30 mL min−1). | ||
The enantioselectivity observed in this reaction can be explained as follows:17 (1) Asymmetric activation of i-Pr2Zn by the chiral mesoporous silica induces the formation of a chiral zinc species that enables the enantioselective addition to aldehyde 1. (2) Enantiotopic face selective adsorption of the achiral pyrimidine-5-carbaldehyde 1 to the helical surface of the chiral mesoporous silica enables the addition of i-Pr2Zn onto the preferred face. (3) Enantioselective adsorption of the initially formed zinc alkoxide of pyrimidyl alkanol 2 induces an enantiomeric imbalance in the reaction solution. Then, the induced enantiomeric imbalance is amplified to provide the overwhelming enantioenrichment of product 2 by subsequent asymmetric autocatalysis.8b
In conclusion, a highly enantioselective addition of diisopropylzinc to pyrimidine-5-carbaldehyde 1 was achieved to afford a highly enantioenriched organic compound by utilizing chiral mesoporous silica as a source of chirality in conjunction with asymmetric autocatalysis. We have clearly demonstrated that helical mesoporous silica has the capability, due to its large reactive surface, to be responsible for the enantioselective addition of i-Pr2Zn to pyrimidine-5-carbaldehyde 1. We believe that these results show the potential for using the large reactive surface of chiral mesoporous silica for asymmetric reactions as a chiral inorganic catalyst.
This work was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS) and by MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016.
Notes and references
- (a) Catalytic Asymmetric Synthesis, ed. I. Ojima, John Wiley & Sons Inc, Hoboken, New Jersey, 3rd edn, 2010 Search PubMed; (b) Comprehensive Asymmetric Catalysis I-III, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer-Verlag, Berlin, 1999 Search PubMed.
- (a) C. E. Song and S.-g. Lee, Chem. Rev., 2002, 102, 3495–3524 CrossRef CAS PubMed; (b) M. Heitbaum, F. Glorius and I. Escher, Angew. Chem., Int. Ed., 2006, 45, 4732–4762 CrossRef CAS PubMed and references cited therein.
- (a) P. McMorn and G. J. Hutchings, Chem. Soc. Rev., 2004, 33, 108–122 RSC; (b) K. Soai, M. Watanabe and A. Yamamoto, J. Org. Chem., 1990, 55, 4832–4835 CrossRef CAS; (c) K. C. W. Chong, J. Sivaguru, T. Shichi, Y. Yoshimi, V. Ramamurthy and J. R. Scheffer, J. Am. Chem. Soc., 2002, 124, 2858–2859 CrossRef CAS PubMed; (d) S. Fujita, H. Sato, N. Kakegawa and A. Yamagishi, J. Phys. Chem. B, 2006, 110, 2533–2540 CrossRef CAS PubMed.
- (a) B. Lebeau, A. Galarneau and M. Linden, Chem. Soc. Rev., 2013, 42, 3661–3662 RSC; (b) C. T. Kresge and W. J. Roth, Chem. Soc. Rev., 2013, 42, 3663–3670 RSC; (c) T. Yanagisawa, T. Shimizu, K. Kuroda and C. Kato, Bull. Chem. Soc. Jpn., 1990, 63, 988–992 CrossRef CAS and references cited therein.
- Reviews: (a) J. Y. Ying, C. P. Mehnert and M. S. Wong, Angew. Chem., Int. Ed., 1999, 38, 56–77 CrossRef CAS; (b) M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS PubMed; (c) D. Trong, D. Desplantier-Giscard, C. Danumah and S. Kaliaguine, Appl. Catal., A, 2003, 253, 545–602 CrossRef; (d) T. Yokoi, Y. Kubota and T. Tatsumi, Appl. Catal., A, 2012, 421–422, 14–37 CrossRef CAS PubMed; (e) S. Kwon, R. K. Singh, R. A. Perez, E. A. Abou Neel, H.-W. Kim and W. Chrzanowski, J. Tissue Eng., 2013, 4, 1–19 Search PubMed; (f) N. Gargiulo, F. Pepe and D. Caputo, J. Nanosci. Nanotechnol., 2014, 14, 1811–1822 CrossRef CAS PubMed; (g) L. Wang and F.-S. Xiao, Green Chem., 2015, 17, 24–39 RSC.
- For example, (a) E. Gross, J. H. Liu, S. Alayoglu, M. A. Marcus, S. C. Fakra, F. D. Toste and G. A. Somorjai, J. Am. Chem. Soc., 2013, 135, 3881–3886 CrossRef CAS PubMed; (b) R. Raja, J. M. Thomas, M. D. Jones, B. F. G. Johnson and D. E. W. Vaughan, J. Am. Chem. Soc., 2003, 125, 14982–14983 CrossRef CAS PubMed.
- (a) S. Che, Z. Liu, T. Ohsuna, K. Sakamoto, O. Terasaki and T. Tatsumi, Nature, 2004, 429, 281–284 CrossRef CAS PubMed; (b) T. Yokoi, K. Ogawa, D. Lu, J. Kondo, Y. Kubota and T. Tatsumi, Chem. Mater., 2011, 23, 2014–2016 CrossRef CAS; (c) T. Yokoi, S. Sato, Y. Ara, D. Lu, Y. Kubota and T. Tatsumi, Adsorption, 2010, 16, 577–586 CrossRef CAS.
- (a) K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767–768 CrossRef CAS; (b) I. Sato, H. Urabe, S. Ishiguro, T. Shibata and K. Soai, Angew. Chem., Int. Ed., 2003, 42, 315–317 CrossRef CAS PubMed.
- For the mechanistic analyses of asymmetric autocatalysis see: (a) I. Sato, D. Omiya, H. Igarashi, K. Kato, Y. Ogi, K. Tsukiyama and K. Soai, Tetrahedron: Asymmetry, 2003, 14, 975–979 CrossRef CAS; (b) M. Quaranta, T. Gehring, B. Odell, J. M. Brown and D. G. Blackmond, J. Am. Chem. Soc., 2010, 132, 15104–15107 CrossRef CAS PubMed; (c) G. Ercolani and L. Schiaffino, J. Org. Chem., 2011, 76, 2619–2626 CrossRef CAS PubMed; (d) J.-C. Micheau, C. Coudret, J.-M. Cruz and T. Buhse, Phys. Chem. Chem. Phys., 2012, 14, 13239–13248 RSC; (e) T. Gehring, M. Quaranta, B. Odell, D. G. Blackmond and J. M. Brown, Angew. Chem., Int. Ed., 2012, 51, 9539–9542 CrossRef CAS PubMed; (f) I. D. Gridnev and A. K. Vorobiev, ACS Catal., 2012, 2, 2137–2149 CrossRef CAS; (g) I. D. Gridnev and A. K. Vorobiev, Bull. Chem. Soc. Jpn., 2015, 88, 333–340 CrossRef.
- Reviews by us: (a) K. Soai, T. Kawasaki and A. Matsumoto, Acc. Chem. Res., 2014, 47, 3643–3654 CrossRef CAS PubMed; (b) T. Kawasaki and K. Soai, Bull. Chem. Soc. Jpn., 2011, 84, 879–892 CrossRef CAS; (c) K. Soai, T. Kawasaki and A. Matsumoto, Chem. Rec., 2014, 14, 70 CrossRef CAS PubMed; (d) T. Kawasaki, I. Sato, H. Mineki, A. Matsumoto and K. Soai, Yuki Gosei Kagaku Kyokaishi, 2013, 71, 109–123 CrossRef CAS; (e) K. Soai and T. Kawasaki, Top. Organomet. Chem., 2013, 44, 261–280 CrossRef CAS; (f) T. Kawasaki and K. Soai, Isr. J. Chem., 2012, 52, 582–590 CrossRef CAS.
- Reviews by others: (a) J.-K. Micheau, C. Coudret and T. Buhse, in The Soai Reaction and Related Topic, ed. G. Palyi, C. Zucchi and L. Caglioti, Accad. Nazl. Sci. Lett. Arti, Artestampa, Modena, 2012, pp. 169–196 Search PubMed; (b) T. Gehring, M. Busch, M. Schlageter and D. Weingand, Chirality, 2010, 22, E173–E182 CrossRef CAS PubMed; (c) B. Barabas, J. Toth and G. Palyi, J. Math. Chem., 2010, 48, 457–489 CrossRef CAS; (d) G. Lente, Symmetry, 2010, 2, 767–798 CrossRef CAS PubMed; (e) J. M. Brown, I. Gridnev and J. Klankermayer, Top. Curr. Chem., 2008, 284, 35–65 CrossRef CAS; (f) L. Caglioti and G. Palyi, Chim. Oggi, 2008, 26, 41–42 CAS; (g) A. J. Bissette and S. P. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800–12826 CrossRef CAS PubMed; (h) J. Podlech and T. Gehring, Angew. Chem., Int. Ed., 2005, 44, 5776–5777 CrossRef CAS PubMed.
- Asymmetric autocatalysis initiated with chiral inorganic materials such as amorphous silica, silsesquioxane, synthetic hectorite, sodium chlorate and cinnabar, see: (a) I. Sato, K. Kadowaki, H. Urabe, J. Hwa Jung, Y. Ono, S. Shinkai and K. Soai, Tetrahedron Lett., 2003, 44, 721–724 CrossRef CAS; (b) T. Kawasaki, K. Ishikawa, H. Sekibata, I. Sato and K. Soai, Tetrahedron Lett., 2004, 45, 7939–7941 CrossRef CAS PubMed; (c) T. Kawasaki, T. Omine, K. Suzuki, H. Sato, A. Yamagishi and K. Soai, Org. Biomol. Chem., 2009, 7, 1073–1075 RSC; (d) I. Sato, K. Kadowaki and K. Soai, Angew. Chem., Int. Ed., 2000, 39, 1510–1512 CrossRef CAS; (e) H. Shindo, Y. Shirota, K. Niki, T. Kawasaki, K. Suzuki, Y. Araki, A. Matsumoto and K. Soai, Angew. Chem., Int. Ed., 2013, 52, 9135–9138 CrossRef CAS PubMed.
- D and L-Quartz (SiO2) promoted asymmetric autocatalysis see: K. Soai, S. Osanai, K. Kadowaki, S. Yonekubo, T. Shibata and I. Sato, J. Am. Chem. Soc., 1999, 121, 11235–11236 CrossRef CAS.
- Spontaneous absolute asymmetric synthesis mediated by achiral silica gel, see: T. Kawasaki, K. Suzuki, M. Shimizu, K. Ishikawa and K. Soai, Chirality, 2006, 18, 479–482 CrossRef CAS PubMed.
- (a) T. Kawasaki, Y. Matsumura, T. Tsutsumi, K. Suzuki, M. Ito and K. Soai, Science, 2009, 324, 492–495 CrossRef CAS PubMed; (b) T. Kawasaki, H. Tanaka, T. Tsutsumi, T. Kasahara, I. Sato and K. Soai, J. Am. Chem. Soc., 2006, 128, 6032–6033 CrossRef CAS PubMed; (c) T. Kawasaki, C. Hohberger, Y. Araki, K. Hatase, K. Beckerle, J. Okuda and K. Soai, Chem. Commun., 2009, 5621–5623 RSC; (d) T. Kawasaki, Y. Okano, E. Suzuki, S. Takano, S. Oji and K. Soai, Angew. Chem., Int. Ed., 2011, 50, 8131–8133 CrossRef CAS PubMed.
- The direction of asymmetric induction has been examined employing N-myristoyl-D-alanine and its L-isomer as chiral initiators of asymmetric autocatalysis, respectively (see the ESI†).
- The computational mechanism of enantioselection on the surface of chiral crystal in asymmetric autocatalysis: D. J. Carter, A. L. Rohl, A. Shtukenberg, S. Bian, C. Hu, L. Baylon, B. Kahr, H. Mineki, K. Abe, T. Kawasaki and K. Soai, Cryst. Growth Des., 2012, 12, 2138–2145 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc01750e |
| ‡ Present address: Department of Materials Science and Engineering, University of Fukui, Bunkyo, Fukui, 910-8507, Japan. |
| This journal is © The Royal Society of Chemistry 2015 |