Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A rationally designed peptidomimetic biosensor for sialic acid on cell surfaces†
Preeti Madhukar
Chaudhary‡
,
Raghavendra Vasudeva
Murthy‡
,
Rohan
Yadav
and
Raghavendra
Kikkeri
*
Indian Institute of Science Education and Research, Dr. Homi Bhabbha Road, Pashan, Pune 411008, India. E-mail: rkikkeri@iiserpune.ac.in; Fax: +91-20-25899790; Tel: +91-20-25908207
First published on 2nd April 2015
Abstract
We have developed peptidomimetic sialic acid (Sia) biosensors using boronic acid and arginine groups on the peptide backbone. The designed peptides were conjugated to fluorescent streptavidin via biotin enabling the optical labeling of cells. This approach provides unique opportunities to detect Sia composition on the cell surfaces and filopodia.
Proteomic, genomic and glycomic changes occur during carcinogenesis resulting in an alteration of cellular features. The differences in the surface profiles of malignant and their non-malignant counterparts can serve as a molecular address for drug delivery to the desired cell types.1 Over the last 30 years, dozens of tumor associated carbohydrate antigens (TACA) have been identified and most of these TACAs have sialic acids (Sias) as terminal sugars.2 Nearly 50 different types of naturally occurring Sias have been identified. N-Acetylneuraminic acid (Neu5Ac) and its hydroxylated derivative, N-glycolylneuraminic acid (Neu5Gc), are the two predominant Sia forms in most mammals.3 Interestingly, larger amounts of Neu5Gc were reported in human malignant tumors and in fetal tissues.4 Furthermore, a significant difference exists between sialylation patterns and concentration in normal cells and their malignant counterparts. Abnormal Sia expression favours cancer metastasis by enhancing migration and tissue invasion.5 It is therefore desirable to develop specific markers for sialic acid glycans.
Several biomacromolecules such as lectins, antibodies or nucleic acids have been used to bind Sias on cancer cells.6 Phage display technology has also been used to develop short 12 or 15-mer peptides of the Sias binding site.7 However, these biomolecules must overcome different drawbacks such as toxicity, selectivity, stability, immunogenicity and cost. Ideally, short peptidomimetics with high affinity and specificity could provide an alternative to lectins. In this context, the ability of boric and phenylboronic acid to interact with sugars has been extensively reported.8 Phenylboronic acid binds covalently to cis 1,2 or 1,3-diols and forms cyclic esters.9 Hall et al. have demonstrated that benzoboroxole conjugated peptidomimetics can be used to target pyranoside sugars.10 Wang et al. synthesized a large library of boronoic acid modified DNAs to target glycoproteins.11 Lavigne et al. used a boronic acid based peptide library to detect glycoproteins.12 Levonis et al. have reported an artificial fluorescent boronate receptor for the detection of free sialic acid.13 Gold coated surfaces,14 glass beads,15 graphene,16 polymers,17 lanthanide complexes18 and quantum dots19 were all used as templates to conjugate boronoic acid derivatives to target cell surface Sias. However, interaction between phenylboronic acid and Sias in glycoconjugates is much weaker than free Neu5Ac, due to the presence of glycosidic linkage at the C2 position. To increase the avidity of specific peptide–sugar interactions multivalent guanidine or tryptophan moieties were incorporated as secondary ligation groups.20 However, the selectivity and sensitivity of these molecules are still far lower compared to natural lectins. Herein, we propose to synthesize a new class of peptidomimetics based on a short hexapeptide model (SPYGRC), which has been reported to bind Sias containing glycoconjugates.21 We have synthesized peptidomimetic analogs by incorporating phenylboronic acid (meta or para-orientation) residues into the EYGR tetrapeptide. Molecular modelling studies have revealed boronic acid and arginine groups in close proximity (Fig. S22, ESI†). We hypothesized that such close proximity could allow selective binding of phenylboronic acid with germinal diol function at C8 and C9 of Sia as well as electrostatic interactions between positively charged arginine and carboxylic acid residues of Sia. In addition, the tyrosine amino acid residue was expected to provide H-bonding and CH–π interaction to stabilize the pyranoside ring of Sia. The binding affinity with different monosaccharides was achieved by microarray. Confocal imaging of cancer cell lines – HeLa (cervix) and MDA-MB-231 (breast) and normal cell line – NIH-3T3 (fibroblast) revealed the correlation of the binding pattern of peptidomimetics and Sambucus Nigra Lectin (SNA), a commercial sialic acid binding lectin.
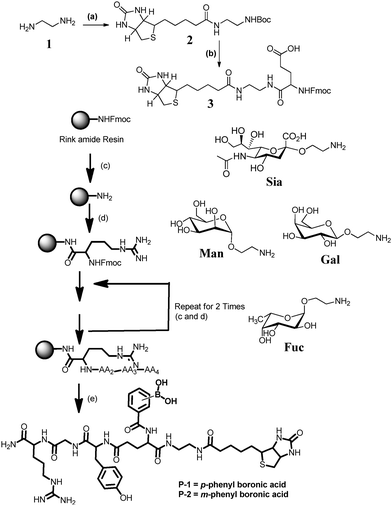
The synthesis of peptides P-1 and P-2 was done using Fmoc solid phase peptide synthesis on Rink amide resin (Fig. 1). Both peptidomimetics are composed of the same amino acid sequence with a terminal biotin residue, however, they differ in the m or p-phenyl boronic acid residue. The biotin residue provided an additional slot for conjugation with fluorescent-streptavidin. P-1 was synthesized by step-by-step coupling of biotin conjugated FmocGlu(OtBu)OH (3), FmocGlyOH, FmocTyr(tBu)OH and FmocArg(Pbf)OH amino acid residues. Finally, m or p-phenyl boronic acid was coupled to the glutamic acid residue to obtain the final peptides (Fig. 1). After cleavage from the resin, the peptides were purified by preparative HPLC and characterized by HRMS, 1H and 13C-NMR.
Next, we examined the interaction of the peptidomimetics with sugars. Four monosaccharides (Sia, Man, Gal, and Fuc) were printed in eight (50, 10, 5, 2, 1, 0.5, 0.2 and 0.1 μM) different concentrations on the N-hydroxysuccinimide derivatized surfaces. The resulting sugar arrays were treated with P-1, P-2 and SNA in the presence of 0.05% Tween-20, followed by Cy3-streptavidin. Mean fluorescence intensities were determined by using data from five independent experiments. Results obtained from sugar array show that P-1 displays a strong interaction with Sia and a weak interaction with Man, Gal and Fuc sugars (Fig. 2). To understand the molecular level details of these interactions, minimum energy structure was constructed using Sia–P-1 conjugation (Fig. S23a and Table S2, ESI†). To simplify the docking studies, the phenylboronic acid residue was covalently conjugated to C8 and C9 of Sia. As expected, p-orientation of the boronic acid residue provided enough flexibility to accommodate ionic interaction between positively charged arginine and negatively charged carboxylic acid residues of Sia, resulting in selective binding. Despite high reactivity between phenylboronic acid and cis-1,2 or 1,3 diols of other sugars, the differential labeling with other sugars is likely related to the slow reactivity (Fig. S24 and Table S3, ESI†). In the case of the P-2 peptide, microarray studies showed an identical binding pattern with all sugars. Molecular modelling of the P-2–sialic acid complex clearly showed close proximity between arginine–phenylboronic acid ester at the same time when the carboxylic acid residue of the Sia ligand is far away from the arginine residue (Fig. S23b, ESI†). A similar experiment with SNA showed selective binding to Sia compared to other sugars. These results indicated that P-1 might be a better candidate to sense sialic acid compared to P-2 analogs.
Microarray results can also be utilized qualitatively and quantitatively for the analysis of specific interactions and it is considered as a powerful method for the rapid and simultaneous measurement of dissociation constants (Kd).22 Thus, sugar microarrays were utilized to determine Kd values for peptidomimic–Sia interaction. The Sia ligand was imprinted eight times with different concentrations on N-hydroxysuccinimide derivatized surfaces, and the peptide microarrays were then probed with four concentrations each of P-1 and P-2 peptides with eight different concentrations of sugars, and the Kd values were determined (Table 1 and Table S1 and Fig. S7 to S12, ESI†). The results showed that most of the curves fit well to one site binding. The observations showed that both the peptides P-1 and P-2 were bound to Sia with sub-micromolar Kd values and display a very weak binding with other sugars. Moreover, Sia–P-1 was relatively strongly bound compared to Sia–P-2 with a Kd value close to SNA (Fig. 3).
| Sugar | P-1 (μM) | P-2 (μM) |
|---|---|---|
| Sialic acid (Sia) | 0.0754 | 0.0786 |
| Mannose (Man) | 10.23 | 0.104 |
| Galactose (Gal) | 2030 | 0.0753 |
| Fucose (Fuc) | 10.56 | 0.127 |
 | ||
| Fig. 3 Fluorescence image of four different concentrations of P-1 peptides with eight different concentrations of sialic acid (Sia), fucose (Fuc), galactose (Gal) and mannose (Man). | ||
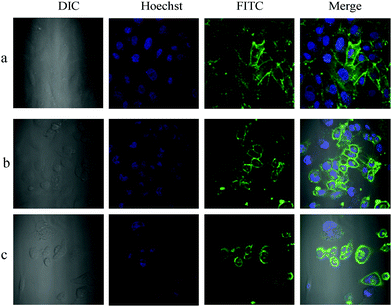
Using microscopic imaging, P-1 was evaluated for binding with Sias on normal and cancer cell surfaces, which are known to express different levels of Sias on the cell membrane. In the first step, P-1 was conjugated with FITC-streptavidin (F-S) by mixing the two in PBS pH 7.4 for 2 h at RT. As can be seen from confocal images, both cancer cells and normal cells were visibly stained after 30 min incubation with the P-1(F-S) conjugate (Fig. 4 and Fig. S13, S15 and S17, ESI†).
Based on the morphology of the cells, P-1 appears decorated on the cell surfaces and was found to internalize to some extent (Fig. S14, S16 and S18, ESI†). These results correlate with that of Liu et al. obtained with QD–phenylboronic acid conjugates.23 To further validate that the staining observed was indeed due to the specific recognition between Sia and P-1, Sias were chopped off from the HeLa cell surfaces by treating them with sialidase enzyme for 30 min. It was clearly evident that the removal of Sias effectively inhibited P-1 binding on the cell surfaces (Fig. S20, ESI†). A similar Sia distribution pattern was also revealed with Sia-specific FITC labelled SNA lectin (Fig. S19, ESI†). Also, Sia composition on normal and cancer cell types was evaluated by measuring the average fluorescence intensity. As expected P-1(F-S) conjugate treated HeLa cells showed 35–40% strong fluorescence response compared to that of normal (NIH-3T3) cells (Fig. S25, ESI†). In contrast, MDA-MB-231 showed nearly 36–42% less fluorescence response compared to HeLa. A similar experiment with FITC-conjugated SNA revealed identical results. Accordingly, we suspect that breast cancer cells might express less Sias compared to cervix or may express Sias in other linkage forms, which might be difficult to identify with the SNA and/or P-1 peptide.
A close examination of P-1 and SNA stained HeLa showed that both conjugates also stained filopodia (Fig. 5 and Fig. S19b, ESI†), the connecting networks between the cells.24 To further confirm that the staining of filopodia is due to specific Sia glycan, we performed confocal imaging with FITC conjugated Concanavalin A, peanut agglutinin (PNA) and Ulex europaeus agglutinin 1 (UEA1), where Concanavalin A binds to all D-mannose and D-glucose structures, PNA is specific to T-antigen, a galactosyl (β1–3) N-acetylgalactosamine structure, and UEA1 is specific to the α-linked L-fucose residue. HeLa cells were treated with all these lectins separately and imaged. Interestingly, HeLa displayed a high concentration of mannose terminated glycans and a small quantity of fucose, galactose or galactosamine terminal sugars. However, none of them stained the filopodia component, indicating the presence of Sia glycans in the filopodia region (Fig. S21, ESI†). In the case of MDA-MB-231, the filopodia level was very less to get distinct staining (Fig. S17, ESI†).
In summary, we have shown how a short peptidomimetic can be applied to diagnose cell surface Sias. Microarray and confocal imaging studies clearly showed selective interactions between P-1 and Sias. Our results clearly show the presence of Sias on filopodia, which is involved in cell motility, proliferation and cell–cell interactions. In a more general perspective, the ease and low cost of synthesis make P-1 an ideal alternative substrate for SNA lectin to analyze complex glycocalyx machinery.
R. K thanks IISER Pune, Indo-German (DST-MPG) program and UGC, India, for financial support.
Notes and references
- (a) S. Hanash, Nat. Rev. Cancer, 2004, 4, 638–644 CrossRef CAS PubMed; (b) M. A. Hollingsworth and B. J. Swanson, Nat. Rev. Cancer, 2004, 4, 45–60 CrossRef CAS PubMed; (c) J. D. Wulfkunhle, L. A. Liotta and E. F. Petricoin, Nat. Rev. Cancer, 2003, 3, 267–275 CrossRef PubMed; (d) V. Padler-Karavani, N. Hurtado-Ziola, M. Pu, H. Yu, S. Huang, S. Muthana, H. A. Chokhawala, H. Cao, P. Secrest, D. Friedmann-Morvinski, O. Singer, D. Ghaderi, I. M. Verma, Y.-T. Liu, K. Messer, X. Chen, A. Varki and R. Schwab, Cancer Res., 2011, 71, 3352–3363 CrossRef CAS PubMed.
- (a) T. Buskas, P. Thompson and G.-J. Boons, Chem. Commun., 2009, 5335–5349 RSC; (b) R. M. Wilson and S. J. Danishefsky, J. Am. Chem. Soc., 2013, 135, 14462–14472 CrossRef CAS PubMed.
- (a) T. Angata and A. Varki, Chem. Rev., 2002, 102, 439–469 CrossRef CAS PubMed; (b) A. Varki, R. D. Cummings, J. D. Esko, H. H. Freeze, P. Stanley, C. R. Bertozzi, G. W. Hart and M. E. Etzler, Essentials of Glycobiology, Cold Spring Harbor Press, Cold Spring Harbor, NY, 2nd edn, 2009, pp. 199–218 Search PubMed; (c) X. Chen and A. Varki, ACS Chem. Biol., 2010, 5, 163–176 CrossRef CAS PubMed; (d) R. Kikkeri, V. Padler-Karavani, S. Diaz, A. Verhagen, H. Yu, H. Cao, M. A. Langereis, R. J. De Groot, X. Chen and A. Varki, Anal. Chem., 2013, 85, 3864–3870 CrossRef CAS.
- (a) P. Tangvoranuntakul, P. Gagneux, S. Diaz, M. Bardor, N. Varki, A. Varki and E. Muchmore, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 12045–12050 CrossRef CAS PubMed; (b) M. Hedlund, V. Padler-Karavani, N. M. Varki and A. Varki, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18936–18941 CrossRef CAS PubMed; (c) R. E. Taylor, C. J. Gregg, V. Padler-Karavani, D. Ghaderi, H. Yu, S. Huang, R. U. Sorensen, X. Chen, J. Inostroza, V. Nizet and A. Varki, J. Exp. Med., 2010, 207, 1637–1646 CrossRef CAS PubMed; (d) A. Varki, Glycoconjugate J., 2009, 26, 231–245 CrossRef CAS PubMed.
- (a) Y. J. Kim and A. Varki, Glycoconjugate J., 1997, 14, 569–576 CrossRef CAS; (b) A. Cazet, S. Julien, M. Bobowski, M. A. Krzewinski-Recchi, A. Harduin-Lepers, S. Groux-Degroote and P. Delannoy, Carbohydr. Res., 2010, 345, 1377–1383 CrossRef CAS PubMed.
- (a) I. J. Goldstein and C. E. Hayes, Adv. Carbohydr. Chem. Biochem., 1978, 35, 127–340 CrossRef CAS; (b) M. Ambrosi, N. R. Cameron and B. G. Davis, Org. Biomol. Chem., 2005, 3, 1593–1608 RSC.
- (a) S. Hakomori, Adv. Exp. Med. Biol., 2001, 491, 369–402 CrossRef CAS; (b) P. Verheesen and T. Laeremans, Methods Mol. Biol., 2012, 991, 81–104 Search PubMed; (c) G. Castel, M. Chtéoui, B. Heyd and N. Tordo, Molecules, 2011, 16, 3499–3518 CrossRef CAS PubMed.
- (a) S. Jin, Y. Cheng, S. Reid, M. Li and B. Wang, Med. Res. Rev., 2010, 30, 171–257 CAS; (b) Y. Zou, D. L. Broughton, K. L. Bicker, P. R. Thompson and J. J. Lavigne, ChemBioChem, 2007, 8, 2048–2051 CrossRef CAS PubMed; (c) L. N. Neupane, S. Y. Han and K.-H. Lee, Chem. Commun., 2014, 50, 5854–5857 RSC; (d) T. L. Halo, J. Appelbaum, E. M. Hobert, D. M. Balkin and A. Schepartz, J. Am. Chem. Soc., 2009, 131, 438–439 CrossRef CAS PubMed; (e) X.-D. Xu, H. Cheng, W.-H. Chen, S.-X. Cheng, R.-X. Zhuo and X.-Z. Zhang, Sci. Rep., 2013, 3, 2679 Search PubMed; (f) B. Rout, P. Milko, M. A. Iron, L. Motiei and D. Margulies, J. Am. Chem. Soc., 2013, 135, 15330–15333 CrossRef CAS PubMed; (g) T. J. Burnett, H. C. Peebles and J. H. Hageman, Biochem. Biophys. Res. Commun., 1980, 96, 157–162 CrossRef CAS; (h) W. Yang, S. Gao, X. Gao, V. V. R. Karnati, W. Ni, B. Wamg, W. B. Hooks, J. Carson and B. Weston, Bioorg. Med. Chem. Lett., 2002, 12, 2175–2177 CrossRef CAS; (i) P. J. Duggan and D. A. Offermann, Tetrahedron, 2009, 65, 109–114 CrossRef CAS PubMed.
- (a) X. Wu, Z. Li, X.-X. Chen, J. S. Fossey, T. D. James and Y.-B. Jiang, Chem. Soc. Rev., 2013, 42, 8032–8048 RSC; (b) H. Otsuka, E. Uchimura, H. Koshino, T. Okano and K. Kataoka, J. Am. Chem. Soc., 2003, 125, 3493–3502 CrossRef CAS PubMed; (c) H. Li, Y. Liu, J. Liu and Z. Liu, Chem. Commun., 2011, 47, 8169–8171 RSC.
- A. Pal, M. Bérubé and D. G. Hall, Angew. Chem., Int. Ed., 2010, 49, 1492–1495 CrossRef CAS PubMed.
- N. Lin, J. Yan, Z. Huang, C. Altier, M. Li, N. Carrasco, M. Suyemoto, L. Johnston, S. Wang, Q. Wang, H. Fang, J. Caton-Williams and B. Wang, Nucleic Acids Res., 2007, 35, 1222–1229 CrossRef CAS PubMed.
- K. L. Bicker, J. Sun, J. J. Lavigne and P. R. Thompson, ACS Comb. Sci., 2011, 13, 232–243 CrossRef CAS PubMed.
- S. M. Levonis, M. J. Kiefel and T. A. Houston, Chem. Commun., 2009, 2278–2280 RSC.
- (a) B. S. Kwaka, H. O. Kimb, J. H. Kimc, S. Leec and H.-I. Junga, Biosens. Bioelectron., 2012, 35, 484–488 CrossRef PubMed; (b) J. Macalindong, D. Guzman, S. A. Soper and R. L. McCarley, Anal. Chem., 2010, 82, 8970–8977 CrossRef PubMed; (c) M. J. Linman, H. Yu, X. Chen and Q. Cheng, ACS Appl. Mater. Interfaces, 2009, 1, 1755–1762 CrossRef CAS PubMed.
- M. D. Sørensen, R. Martins and O. Hindsgaul, Angew. Chem., Int. Ed., 2007, 46, 2403–2407 CrossRef PubMed.
- Y. Zhou, H. Dong, L. Liu, J. Liu and M. Xu, Biosens. Bioelectron., 2014, 60, 231–236 CrossRef CAS PubMed.
- (a) A. Kugimiya and T. Takeuchi, Biosens. Bioelectron., 2001, 16, 1059–1062 CrossRef CAS; (b) L. Song, J. Zhao, S. Luan, J. Ma, J. Liu, X. Xu and J. Yin, ACS Appl. Mater. Interfaces, 2013, 5, 13207–13215 CrossRef CAS PubMed; (c) G. Pan, B. Guo, Y. Ma, W. Cui, F. He, B. Li, H. Yang and K. J. Shea, J. Am. Chem. Soc., 2014, 136, 6203–6206 CrossRef CAS PubMed; (d) S. Deshayes, H. Cabral, T. Ishii, Y. Miura, S. Kobayashi, T. Yamashita, A. Matsumoto, Y. Miyahara, N. Nishiyama and K. Kataoka, J. Am. Chem. Soc., 2013, 135, 15501–15507 CrossRef CAS PubMed; (e) V. L. de Talancé, O. Massinon, R. Baati, A. Wagnerb and S. P. Vincent, Chem. Commun., 2012, 48, 10684–10686 RSC.
- (a) L. Frullano, J. Rohovec, S. Aime, T. Maschmeyer, M. I. Prata, J. J. de Lima, C. F. Geraldes and J. A. Peters, Chem. – Eur. J., 2004, 10, 5205–5217 CrossRef CAS PubMed; (b) M. Regueiro-Figueroa, K. Djanashvili, D. Esteban-Gómez, T. Chauvin, É. Tóth, A. de Blas, T. Rodríguez-Blas and C. Platas-Iglesias, Inorg. Chem., 2010, 49, 4212–4223 CrossRef CAS PubMed; (c) S. G. Crich, D. Alberti, I. Szabo, S. Aime and K. Djanashvili, Angew. Chem., Int. Ed., 2013, 52, 1161–1164 CrossRef PubMed.
- W. Zhang, X.-W. He, Y.-Q. Yang, W.-Y. Li and Y.-K. Zhang, J. Mater. Chem. B, 2013, 1, 347–352 RSC.
- K. Djanashvili, L. Frullano and J. A. Peters, Chem. – Eur. J., 2005, 11, 4010–4018 CrossRef CAS PubMed.
- L. D. Heerze, R. H. Smith, N. Wang and G. D. Armstrong, Glycobiology, 1995, 5, 427–433 CrossRef CAS PubMed.
- J. Pai, T. Yoon, N. D. Kim, I.-S. Lee, J. Yu and I. Shin, J. Am. Chem. Soc., 2012, 134, 19287–19296 CrossRef CAS PubMed.
- A. Liu, S. Peng, J. C. Soo, M. Kuang, P. Chen and H. Duan, Anal. Chem., 2011, 83, 1124–1130 CrossRef CAS PubMed.
- J. Albuschies and V. Vogel, Sci. Rep., 2013, 3, 1658 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc01662b |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2015 |