 Open Access Article
Open Access ArticleA polyamine-modified near-infrared fluorescent probe for selective staining of live cancer cells†
Sandra G.
König
,
Simin
Öz
and
Roland
Krämer
*
Universität Heidelberg, Anorganisch-Chemisches Institut, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany. E-mail: Roland.Kraemer@urz.uni-heidelberg.de
First published on 20th March 2015
Abstract
We report the synthesis of novel polyamine-modified near-infrared (NIR) probes, which show excellent water-solubility and good optical properties. One probe was taken up efficiently by living cancer cell lines whereas no staining of the non-cancer cells was observed.
Fluorophores that emit in the NIR wavelength range (700–900 nm) increasingly attract interest for cell and tissue imaging,1–8 as they display reduced background fluorescence and enable deep tissue penetration. In this context, indotricarbocyanines are among the most commonly used fluorophores and enclose the only clinically approved NIR dye indocyanine green, applied as a perfusion marker in, for instance, image guided oncologic surgery and lymph node imaging.9
Recently, the development of new NIR fluorescent probes that allow for tissue-selective staining due to their intrinsic chemical structures – instead of using covalently attached targeting moieties such as peptides or antibodies – has emerged as a powerful new approach.10 Attachment of cationic amino groups substantially increases the water-solubility of polyaromatic fluorophores for application as biomarkers. Several examples have been reported, including spermine-functionalized perylene bisimides,11,12 perylene bisimides with guanidinium-dendrons,13 tetraazaperopyrenes containing heterocycles with quaternized nitrogen atoms,14 multi-cationic BODIPY dyes,15 axially di-substituted silicon phthalocyanines,16 and cyanine dyes.17 Polyamine-modified probes in their protonated form display high affinity to polyanionic biopolymers – such as nucleic acids and glycosaminoglycans – and are used for the detection of these molecules.12,18 In a medical context, an attractive application of polyamine-modified probes is the targeting of cancer cells,19 as most cancer cells express an overactive polyamine transport system (PTS) and tend to accumulate derivatives of the natural polyamines spermine and spermidine.20,21 F14512, a spermine-conjugate of the topoisomerase inhibitor epipodophyllotoxin, has recently completed a phase I clinical trial and is considered as a promising drug candidate for the treatment of acute myeloid leukemia.22 The structural requirements for effective cellular uptake of polyamine probes, however, remain obscure; only few probes are effectively internalized, either independently from the PTS or in strong correlation with the activity of the latter.23
Herein, we describe a new polyamine-modified indotricarbocyanine fluorescent probe with absorption and emission maxima in the near-infrared region. This probe is suitable for fluorescence imaging of living cells due to its high extinction coefficient, sufficient quantum yield, excellent water-solubility, and low cytotoxicity under the conditions applied for staining. Additionally, we show that the probe specifically targets living cancer cell lines whereas no accumulation of the dye is observed in non-cancer cells.
The double carboxylic acid modified indotricarbocyanine dye backbone24,251 allows for highly modular chemical conjugation and was thus selected as synthetic platform. In order to evaluate the influence of different chain lengths on cellular uptake, we prepared a series of dye conjugates containing a variety of natural polyamines.
N-Boc-putrescine, N1,N5-di-boc-spermidine and N1,N4,N9-tri-boc-spermine were coupled to backbone 1via amide-bond formation. Subsequent removal of the protecting groups yielded conjugates 2–4 (Fig. 1a; see ESI† for synthetic details).
 | ||
| Fig. 1 Structures of the herein investigated polyamine-dye conjugates based on (a) chromophore 1 and (b) chromophore 7. Counter ions are omitted. | ||
According to previous reports by Winkler et al.,26 coupling of spermine leads to the functional analogue of a spermidine conjugate due to impairment of one NH2 group. Therefore, we additionally prepared conjugate 5 through attachment of the chemically extended spermine derivative N1,N4,N9,N13-tetra-boc-1,16-diamino-4,9,13-triazahexadecane. The resulting dye-polyamine conjugates 2–5 showed very high extinction coefficients (260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–285
000–285![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and sufficient fluorescence quantum yields (0.10–0.15) in combination with good solubility in aqueous solutions.
000 M−1 cm−1) and sufficient fluorescence quantum yields (0.10–0.15) in combination with good solubility in aqueous solutions.
As a first step to characterize the labeling efficiency of the dyes 2–5 by flow cytometry, we used a cervical carcinoma cell line (HeLa) and treated the cells with 10 μM solutions of the dyes for various amounts of time. The results revealed that conjugate 5 shows the highest staining efficiency while almost no staining was observed for 2 (Fig. S1, ESI†). Therefore, we selected conjugate 5 for further investigations.
For a comprehensive comparison with 5, the related indotricarbocyanine derivatives 6 and 8 (Fig. 1) were also prepared. In order to study the influence of the N-connecting alkyl moieties, we prepared 6 which contains a non-natural linear polyamine with the same number of amino groups as in 5 but with shorter –(CH2)n– fragments. Due to the promising results with the “extended” spermine conjugate 5, we also prepared conjugate 8 (Fig. 1b) which contains the same polyamine but the structurally different indotricarbocyanine core 7 (“cypate”)27 that was chosen due to its analogy to the clinically applied compound indocyanine green (see ESI† for synthetic procedures).
The photophysical properties of 5, 6 and 8 are shown in Table 1 and Fig. 2. Dyes 5 and 6 show very high molar extinction coefficients and sufficient quantum yields which compare well to the values of related indotricarbocyanines.28 In line with the data for indocyanine green,29 the molar absorbance and quantum yield of 8 are significantly lower (Table 1).
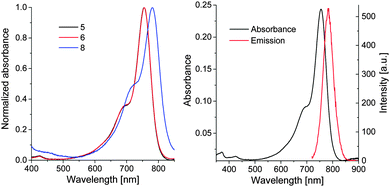 | ||
| Fig. 2 Left: normalized absorption spectra of 5, 6 and 8 in PBS (pH 7.4). Right: absorption and emission spectra of 5 in PBS (pH 7.4). | ||
In order to investigate the applicability of the polyamine-modified fluorophores in biological samples, we determined the cytotoxicity of the dyes in cells. Therefore, we treated HeLa cells with various concentrations (0 nM to 10 μM) of 5 and 8 for 24 h at 37 °C and measured the intracellular ATP levels.31 This yielded IC50 values at 5 μM, demonstrating the weak cellular response of the dyes (Fig. S2, ESI†).
Next, to examine the labeling efficiency of the dyes in living cells, we used various cancer (HeLa and H1650) and non-cancer (hTERT RPE-1 and HDFa) cell lines. Following Hoechst 33342 staining, we incubated the above-mentioned living cells with 10 μM of dye 5 for 5 min at 37 °C and imaged them by fluorescence microscopy. We observed strong staining of the cancer cell lines (Fig. 3). In contrast, in the non-cancer cells almost no accumulation of the dye was detected (Fig. 3), suggesting the function of dye 5 as a cancer-targeting fluorophore. In addition, as anticipated, derivative 6, which contains the non-natural linear polyamine, did not stain any of the tested cell lines (Fig. S3, ESI†).
In order to compare the staining of the cancer and non-cancer cell lines simultaneously, we mixed stably GFP-expressing HeLa cells with HDFa cells in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Following Hoechst 33342 staining, we incubated the cell mixture with 10 μM of 5 for 5 min at 37 °C and imaged by fluorescence microscopy. We observed an almost 100% co-localization (Fig. 4c) of GFP signals coming from HeLa cells (green, Fig. 4a) with dye 5 (red, Fig. 4b). On the other hand, HDFa cells, which can be seen with their nuclei staining (blue), did not accumulate 5 (Fig. 4b), as previously observed (Fig. 3).
1 ratio. Following Hoechst 33342 staining, we incubated the cell mixture with 10 μM of 5 for 5 min at 37 °C and imaged by fluorescence microscopy. We observed an almost 100% co-localization (Fig. 4c) of GFP signals coming from HeLa cells (green, Fig. 4a) with dye 5 (red, Fig. 4b). On the other hand, HDFa cells, which can be seen with their nuclei staining (blue), did not accumulate 5 (Fig. 4b), as previously observed (Fig. 3).
In view of potential applications of the dyes in vivo or in blood samples, we incubated HeLa and hTERT RPE-1 cells with solutions of 5 and 8 in pooled human plasma. For 5, we observed a more than five times higher staining efficiency for the cancer cells compared to the non-cancer cells (p = 0.009) (Fig. 5a). In contrast, 8 did not show a pronounced selectivity for one cell line over the other. Similar results were also observed for 8 by fluorescence microscopy (Fig. S4, ESI†).
Next, we aimed to clarify the role of an overexpressed PTS in the selective uptake of 5 in cancer cells. Following the inhibition of the PTS in HeLa with benzylviologen32 for 24 hours, we incubated the cells with 5 and measured the fluorescence intensity by flow cytometry. Inhibition of the PTS resulted in a 62% of reduction of uptake of 5, compared to untreated cells (p = 0.002) (Fig. 5b), confirming the major role of PTS in the uptake of this particular polyamine dye.
In this work, a series of polyamine-modified NIR-fluorescent probes that exhibit advantageous optical properties and good water-solubility were developed. The most interesting candidates contain an “extended” spermine moiety and were subject of further investigations within living cells. While all of the investigated derivatives proved to have low cytotoxicity under the above-mentioned treatment conditions, only 5 showed enhanced staining of live cancer cell lines whereas non-cancer cells remained unstained. Additionally, we were able to demonstrate the selective staining of cancer cells in human plasma. In order to broaden the applicability of our system, future experiments will focus on systematic investigations with a variety of cell lines.
With regard to its chemical and optical properties, we consider the development of 5 as an important step towards a simple small-molecule probe that combines the polyamine-vector triggered, selective uptake by live cancer cells with minimized interference with cellular autofluorescence due to NIR fluorescence. Such probes may facilitate the fluorescence scanning microscopic identification of individual tumor cells in the presence of a large excess of other cells, as required for the quantification of circulating tumor cells in blood samples33 for early diagnosis.
SGK is grateful to the Stiftung der deutschen Wirtschaft (sdw) for a PhD fellowship.
The authors thank Dr Tautvydas Lisauskas, Dr Holger Erfle, Dominik Brox and Prof. Dirk-Peter Herten for helping with fluorescence microscopy, Elisabeth Treutlein and Ana Lucía Meza Chincha for synthetic support and Berati Cerikan for the hTERT RPE-1 and HeLa T-REX stable LAPtag cell lines.
Notes and references
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- K. Umezawa, D. Citterio and K. Suzuki, Anal. Sci., 2014, 30, 327–349 CrossRef CAS.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- G. Lukinavičius, K. Umezawa, N. Olivier, A. Honigmann, G. Yang, T. Plass, V. Mueller, L. Reymond, I. R. Corrêa Jr, Z.-G. Luo, C. Schultz, E. A. Lemke, P. Heppenstall, C. Eggeling, S. Manley and K. Johnsson, Nat. Chem., 2013, 5, 132–139 CrossRef PubMed.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef CAS PubMed.
- M. Bai and D. J. Bornhop, Curr. Med. Chem., 2012, 19, 4742–4785 CrossRef CAS.
- S. Luo, E. Zhang, Y. Su, T. Cheng and C. Shi, Biomaterials, 2011, 32, 7127–7138 CrossRef CAS PubMed.
- X. Tan, S. Luo, D. Wang, Y. Su, T. Cheng and C. Shi, Biomaterials, 2012, 33, 2230–2239 CrossRef CAS PubMed.
- E. M. Sevick-Muraca, Annu. Rev. Med., 2012, 63, 217–231 CrossRef CAS PubMed.
- H. Hyun, M. H. Park, E. A. Owens, H. Wada, M. Henary, H. J. M. Handgraaf, A. L. Vahrmeijer, J. V. Frangioni and H. S. Choi, Nat. Med., 2015, 21, 192–197 CrossRef CAS PubMed.
- S. Rehm, V. Stepanenko, X. Zhang, T. H. Rehm and F. Würthner, Chem. – Eur. J., 2010, 16, 3372–3382 CrossRef CAS PubMed.
- H. Szelke, S. Schübel, J. Harenberg and R. Krämer, Chem. Commun., 2010, 46, 1667–1669 RSC.
- J. Zhou, J. Zhang, Y. Lai, Z. Zhou, Y. Zhao, H. Wang and Z. Wang, New J. Chem., 2013, 37, 2983–2986 RSC.
- L. Hahn, S. Öz, H. Wadepohl and L. H. Gade, Chem. Commun., 2014, 50, 4941–4943 RSC.
- J. Bartelmess and W. W. Weare, Dyes Pigm., 2013, 97, 1–8 CrossRef CAS PubMed.
- Z. Bıyıklıoğlu, Dyes Pigm., 2013, 99, 59–66 CrossRef PubMed.
- Y. Guminski, T. Imbert, S. Pesnel, A. Pillon, A. Le Pape and S. Lerondel, WO 2012/069607 A1, 2012.
- See for example H. Szelke, S. Schübel, J. Harenberg and R. Krämer, Bioorg. Med. Chem. Lett., 2010, 20, 1445–1447 CrossRef CAS PubMed.
- P. M. Cullis, R. E. Green, L. Merson-Davies and N. Travis, Chem. Biol., 1999, 6, 717–729 CrossRef CAS.
- A. G. Palmer and H. M. Wallace, Amino Acids, 2010, 38, 415–422 CrossRef CAS PubMed.
- N. Seiler, J. G. Delcros and J. P. Moulinoux, Int. J. Biochem. Cell Biol., 1996, 28, 843–861 CrossRef CAS.
- A. Kruczinski, A. Pillon, L. Creancier, I. Vandenberghe, B. Gomes, V. Brel, E. Fournier, J.-P. Annereau, E. Currie, Y. Guminski, D. Bonnet, C. Bailly and N. Guilbaud, Leukemia, 2013, 27, 2139–2148 CrossRef PubMed.
- For a systematic study see Y. Guminski, M. Grousseaud, S. Cugnasse, V. Brel, J.-P. Annereau, S. Vispe, N. Guilbaud, J.-M. Barret, C. Bailly and T. Imbert, Bioorg. Med. Chem. Lett., 2009, 19, 2474–2477 CrossRef CAS PubMed.
- E. Terpetschnig, H. Szmacinski, A. Ozinskas and J. Lakowicz, Anal. Biochem., 1994, 217, 197–204 CrossRef CAS PubMed.
- C. Pavlik, N. Biswal, F. Gaenzler, M. Morton, L. Kuhn, K. Claffey, Q. Zhu and M. Smith, Dyes Pigm., 2011, 89, 9–15 CrossRef CAS PubMed.
- J. Winkler, K. Saadat, M. Díaz-Gavilán, E. Urban and C. R. Noe, Eur. J. Med. Chem., 2009, 44, 670–677 CrossRef CAS PubMed.
- S. Achilefu, H. N. Jimenez, R. B. Dorshow, J. E. Bugaj, E. G. Webb, R. R. Wilhelm, R. Rajagopalan, J. Johler and J. L. Erion, J. Med. Chem., 2002, 45, 2003–2015 CrossRef CAS PubMed.
- K. Licha, B. Riefke, V. Ntziachristos, A. Becker, B. Chance and W. Semmler, Photochem. Photobiol., 2000, 72, 392–398 CrossRef CAS.
- O. G. Björnsson, R. Murphy, V. S. Chadwick and S. Björnsson, J. Clin. Chem. Clin. Biochem., 1983, 21, 453–458 Search PubMed.
- R. C. Benson and H. A. Kues, J. Chem. Eng. Data, 1977, 22, 379–383 CrossRef CAS.
- S. P. Crouch, R. Kozlowski, K. J. Slater and J. Flechter, J. Immunol. Methods, 1993, 160, 81–88 CrossRef CAS.
- M. Wolf, W. E. Hull, W. Mier, S. Heiland, U. Bauder-Wüst, R. Kinscherf, U. Haberkorn and M. Eisenhut, J. Med. Chem., 2007, 50, 139–148 CrossRef CAS PubMed.
- C. Shao, C.-P. Liao, P. Hu, C.-Y. Chu, L. Zhang, M. H. T. Bui, C. S. Ng, D. Y. Josephson, B. Knudsen, M. Tighiouart, H. L. Kim, L. W. K. Chung, R. Wang and E. M. Posadas, PLoS One, 2014, 9, e88967 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental data, general experimental methods, compound characterizations. See DOI: 10.1039/c5cc01637a |
| This journal is © The Royal Society of Chemistry 2015 |



