Open Access Article
Open Access ArticleNickel-catalyzed asymmetric transfer hydrogenation of conjugated olefins†
Siyu
Guo
,
Peng
Yang
and
Jianrong (Steve)
Zhou
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371. E-mail: jrzhou@ntu.edu.sg; Fax: +65 67911961
First published on 12th June 2015
Abstract
Asymmetric transfer hydrogenation of electron-deficient olefins is realized with nickel catalysts supported by strongly σ-donating bisphosphines. Deuterium labeling experiments point to a reaction sequence of formate decarboxylation, asymmetric hydride insertion and non-stereoselective protonation of resulting nickel enolates.
Asymmetric hydrogenation is the state-of-the-art example in homogeneous metal catalysis and it is practiced on large scales in the manufacturing of chiral pharmaceuticals and agrochemicals.1 Today, chiral catalysts of noble metals such as Rh,2 Ru3 and Ir4 dominate the field of asymmetric hydrogenation. Expensive noble metals themselves contributed to a fraction of the total cost of hydrogenation processes, in addition to costly chiral bisphosphines. Furthermore, the mining and purification of these rare metals from ores is energy demanding and costly. These metals are produced in only dozens of tons a year worldwide and are very expensive, often thousand-fold more so than abundant metals such as copper and nickel. They are highly toxic to human and ecosystems. The heavy metal residues in pharmaceutical active ingredients must be reduced to ppm levels according to FDA regulations. Waste treatment after catalytic hydrogenation also incurs additional costs. In comparison, base metals like iron, nickel and copper are much cheaper, less toxic or even nontoxic, and are being produced in millions of tons a year.5
Previously, Pfaltz et al. invented cobalt-catalyzed asymmetric reduction using borohydrides.6 Recently, Chirik's group reported cobalt-catalyzed hydrogenation of styrenes and enamides, but the cobalt catalysts were sensitive to air and moisture and the types of olefins that gave high ee were quite limited.7 In recent years, achiral nickel complexes were found to have non-stereoselective hydrogenation activity towards unsaturated bonds.8 Hamada et al. reported nickel-catalyzed hydrogenation of ketone groups of α-amino-β-ketoesters under dynamic kinetic resolution conditions (around 80% ee).9 Recently our group disclosed highly stereoselective nickel catalysts for transfer hydrogenation of enamides and hydrazones having directing groups.10 Formic acid was used as a safe and easy-to-handle hydrogen source. It has a high volume/density of H2 and is a promising hydrogen storage material.11 In recent years, highly efficient metal catalysts were developed for decomposition of formic acid to release H2.12 In comparison, both high-pressure hydrogen gas and liquid are safety hazard during storage, transport and use.
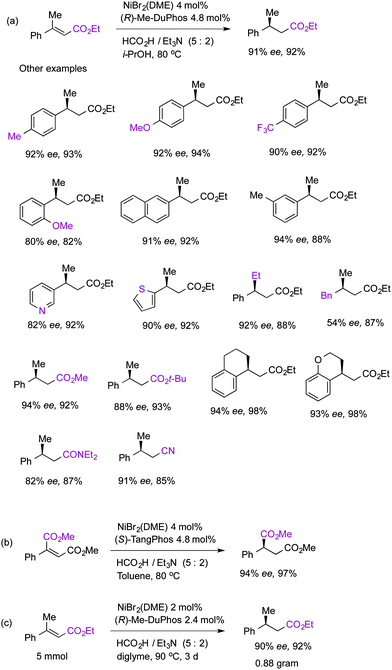
Herein, we report a nickel/DuPhos catalyst for asymmetric transfer hydrogenation of conjugated olefins using formic acid.13 DuPhos, which was invented by Mark Burk, was uniquely active and gave 91% ee value in the model reaction (Fig. 1). The performance of other bisphosphines was unsatisfactory. (S)-Binapine was completely inactive. Other bisphosphine showed little activity, including Ph-BPE, Me-DPF, DuanPhos, QuinoxP*, and Josiphos ligands.14 TangPhos was moderately active.15 Less donating bis(biarylphosphine)s including BINAP, Segphos and DIPAMP were completely inactive. PHOX (Pfaltz ligand) and Feringa's phosphoramidites did not form active catalysts. Iron, cobalt and copper salts were tested with Me-DuPhos and did not form active catalysts.
Isopropanol was used as a solvent in isolation experiments. The nickel catalyst worked well in several other alcohols and diglyme (Table 1). No hydrogenation activity was detected with 10 atm of H2.
(E)-Cinnamates having small β groups cis to the ester groups reacted to afford good ee (Fig. 2). The (Z)-geometric isomer afforded only <20% ee, however. Electron-donating or withdrawing groups on aryl groups were well tolerated, as well as thiophene and pyridine rings. Cyclic olefins were also hydrogenated efficiently to give tetraline derivatives. Furthermore, the ester groups can be replaced by amides and nitriles. α-Phenylmaleate gave 94% ee in the presence of a TangPhos catalyst, while the DuPhos catalyst gave only 65% ee (Fig. 2b). The reaction can be scaled up to gram scale with 2 mol% of nickel (Fig. 2c). In the case of p-chloro-β-methylcinnamate, its aryl-Cl bond was reduced to C–H in the isolated product. It was probably caused by oxidative addition of the C–Cl bond to a nickel(0) species. A β,β-dialkylacrylate was also attempted which afforded a moderate 54% ee. Simple styrene derivatives did not react.
When we attempted the model reaction using [D2]formic acid (Fig. 3a), the β position was fully deuterated as expected. Both α and α′ positions were partially deuterated, too. The deuterium content at α and α′ positions added up to around 100%. Most likely, the main pathway consists of formate decarboxylation on nickel, hydride insertion of the olefin and subsequent non-stereoselective protonation of resulting enolates (Fig. 3a).16 This pathway is distinct from syn-addition of H2 in classical dihydride and monohydride pathways using noble metal catalysts.17
Surprisingly, when we used [D1]formic acid (Fig. 3b), besides deuteration at α position, a significant amount of deuterium ended up in β position (about 30% D). In control experiments, no extra deuteration occurred when the methylcinnamate and its hydrogenation product were treated with [D1]formic acid. Direct hydride transfer from a formate to a metal-bound methylcinnamate via a six-membered transition state is inconsistent with >100% deuterium incorporation.
We propose a minor reaction pathway that involves an equilibrium of (DuPhos)Ni(H)+ and (DuPhos)Ni0via reversible deprotonation. This allows a deuteron of [D1]formic acid to become a nickel deuteride and eventually add to the β position of β-methylcinnamate (Fig. 3c).18 The presence of a nickel(0) species was supported by hydrodechlorination of an aryl C–Cl bond in p-chloro-β-methylcinnamate. As another piece of evidence for nickel(0), when Ni(PPh3)4 was used as a nickel precursor, the model reaction in Table 1 became much slower, but it still afforded 14% conversion after 24 hours at 100 °C.
In summary, we herein report a nickel-catalyzed transfer hydrogenation of conjugated olefins. The nickel catalyst must be supported by a strongly σ-donating bisphosphine, probably for efficient decarboxylation of formate. No directing groups are needed on olefins, unlike enamides and ketone hydrazones that we reported previously.10 Deuterium labelling experiments point to a major pathway involving formate decarboxylation, hydride insertion into olefins and protonation of resulting nickel enolates.
We thank Singapore GSK-EDB green and sustainable manufacturing award and Nanyang Technological University for financial support.
Notes and references
- (a) W. S. Knowles, Angew. Chem., Int. Ed., 2002, 41, 1998 CrossRef CAS; (b) R. Noyori, Adv. Synth. Catal., 2003, 345, 15 CrossRef CAS PubMed; (c) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029 CrossRef CAS PubMed; (d) I. D. Gridnev and T. Imamoto, Acc. Chem. Res., 2004, 37, 633 CrossRef CAS PubMed; (e) X. Cui and K. Burgess, Chem. Rev., 2005, 105, 3272 CrossRef CAS PubMed; (f) V. Farina, J. T. Reeves, C. H. Senanayake and J. J. Song, Chem. Rev., 2006, 106, 2734 CrossRef CAS PubMed; (g) N. B. Johnson, I. C. Lennon, P. H. Moran and J. A. Ramsden, Acc. Chem. Res., 2007, 40, 1291 CrossRef CAS PubMed; (h) A. J. Minnaard, B. L. Feringa, L. Lefort and J. G. De Vries, Acc. Chem. Res., 2007, 40, 1267 CrossRef CAS PubMed; (i) S. J. Roseblade and A. Pfaltz, Acc. Chem. Res., 2007, 40, 1402 CrossRef CAS PubMed; (j) L. A. Saudan, Acc. Chem. Res., 2007, 40, 1309 CrossRef CAS PubMed; (k) H. Shimizu, I. Nagasaki, K. Matsumura, N. Sayo and T. Saito, Acc. Chem. Res., 2007, 40, 1385 CrossRef CAS PubMed; (l) W. Zhang, Y. Chi and X. Zhang, Acc. Chem. Res., 2007, 40, 1278 CrossRef CAS PubMed; (m) D. J. Ager, A. H. M. de Vries and J. G. de Vries, Chem. Soc. Rev., 2012, 41, 3340 RSC; (n) H.-U. Blaser, B. Pugin and F. Spindler, Top. Organomet. Chem., 2012, 1 Search PubMed; (o) J. J. Verendel, O. Pàmies, M. Diéguez and P. G. Andersson, Chem. Rev., 2013, 114, 2130 CrossRef PubMed; (p) J.-P. Genet, T. Ayad and V. Ratovelomanana-Vidal, Chem. Rev., 2014, 114, 2824 CrossRef CAS PubMed.
- Examples: (a) R. Hoen, J. A. F. Boogers, H. Bernsmann, A. J. Minnaard, A. Meetsma, T. D. Tiemersma-Wegman, A. H. M. de Vries, J. G. de Vries and B. L. Feringa, Angew. Chem., Int. Ed., 2005, 44, 4209 CrossRef CAS PubMed; (b) W. Chen, P. J. McCormack, K. Mohammed, W. Mbafor, S. M. Roberts and J. Whittall, Angew. Chem., Int. Ed., 2007, 46, 4141 CrossRef CAS PubMed; (c) K. Dong, Y. Li, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2013, 52, 14191 CrossRef CAS PubMed; (d) Y. Li, K. Dong, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2013, 52, 6748 CrossRef CAS PubMed.
- Examples: (a) T. Ohta, H. Takaya, M. Kitamura, K. Nagai and R. Noyori, J. Org. Chem., 1987, 52, 3174 CrossRef CAS; (b) T. Uemura, X. Zhang, K. Matsumura, N. Sayo, H. Kumobayashi, T. Ohta, K. Nozaki and H. Takaya, J. Org. Chem., 1996, 61, 5510 CrossRef CAS; (c) X. Cheng, Q. Zhang, J.-H. Xie, L.-X. Wang and Q.-L. Zhou, Angew. Chem., Int. Ed., 2005, 44, 1118 CrossRef CAS PubMed.
- Examples: (a) W. Tang, W. Wang and X. Zhang, Angew. Chem., Int. Ed., 2003, 42, 943 CrossRef CAS PubMed; (b) S. Li, S.-F. Zhu, C.-M. Zhang, S. Song and Q.-L. Zhou, J. Am. Chem. Soc., 2008, 130, 8584 CrossRef CAS PubMed; (c) W.-J. Lu, Y.-W. Chen and X.-L. Hou, Angew. Chem., Int. Ed., 2008, 47, 10133 CrossRef CAS PubMed; (d) J. Zhao and K. Burgess, J. Am. Chem. Soc., 2009, 131, 13236 CrossRef CAS PubMed; (e) D. Rageot, D. H. Woodmansee, B. Pugin and A. Pfaltz, Angew. Chem., Int. Ed., 2011, 50, 9598 CrossRef CAS PubMed; (f) S.-F. Zhu, Y.-B. Yu, S. Li, L.-X. Wang and Q.-L. Zhou, Angew. Chem., Int. Ed., 2012, 51, 8872 CrossRef CAS PubMed; (g) M. Bernasconi, M.-A. Müller and A. Pfaltz, Angew. Chem., Int. Ed., 2014, 53, 5385 CrossRef CAS PubMed; (h) Y. Liu, I. D. Gridnev and W. Zhang, Angew. Chem., Int. Ed., 2014, 53, 1901 CrossRef CAS PubMed.
- http://www.infomine.com/investment/metal-prices .
- (a) U. Leutenegger, A. Madin and A. Pfaltz, Angew. Chem., Int. Ed. Engl., 1989, 28, 60 CrossRef PubMed; (b) C. Geiger, P. Kreitmeier and O. Reiser, Adv. Synth. Catal., 2005, 347, 249 CrossRef CAS PubMed.
- (a) S. Monfette, Z. R. Turner, S. P. Semproni and P. J. Chirik, J. Am. Chem. Soc., 2012, 134, 4561 CrossRef CAS PubMed; (b) M. R. Friedfeld, M. Shevlin, J. M. Hoyt, S. W. Krska, M. T. Tudge and P. J. Chirik, Science, 2013, 342, 1076 CrossRef CAS PubMed; (c) R. P. Yu, J. M. Darmon, C. Milsmann, G. W. Margulieux, S. C. E. Stieber, S. DeBeer and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 13168 CrossRef CAS PubMed ; hydroboration: ; (d) L. Zhang, Z. Zuo, X. Wan and Z. Huang, J. Am. Chem. Soc., 2014, 136, 15501 CrossRef CAS PubMed.
- Examples: (a) A. H. Vetter and A. Berkessel, Synthesis, 1995, 419 CrossRef CAS PubMed; (b) I. M. Angulo, A. M. Kluwer and E. Bouwman, Chem. Commun., 1998, 2689 RSC; (c) I. M. Angulo and E. Bouwman, J. Mol. Catal. A: Chem., 2001, 175, 65 CrossRef CAS; (d) S. Kuhl, R. Schneider and Y. Fort, Organometallics, 2003, 22, 4184 CrossRef CAS; (e) A. L. Iglesias and J. J. Garcia, J. Mol. Catal. A: Chem., 2009, 298, 51 CrossRef CAS PubMed; (f) W. H. Harman and J. C. Peters, J. Am. Chem. Soc., 2012, 134, 5080 CrossRef CAS PubMed; (g) K. V. Vasudevan, B. L. Scott and S. K. Hanson, Eur. J. Inorg. Chem., 2012, 4898 CrossRef CAS PubMed; (h) J. Wu, J. W. Faller, N. Hazari and T. J. Schmeier, Organometallics, 2012, 31, 806 CrossRef CAS; (i) T.-P. Lin and J. C. Peters, J. Am. Chem. Soc., 2014, 136, 13672 CrossRef CAS PubMed.
- (a) Y. Hamada, Y. Koseki, T. Fujii, T. Maeda, T. Hibino and K. Makino, Chem. Commun., 2008, 6206 RSC; (b) T. Hibino, K. Makino, T. Sugiyama and Y. Hamada, ChemCatChem, 2009, 1, 237 CrossRef CAS PubMed.
- (a) P. Yang, H. Xu and J. Zhou, Angew. Chem., Int. Ed., 2014, 53, 12210 CrossRef CAS PubMed; (b) H. Xu, P. Yang, P. Chuanprasit, H. Hirao and J. Zhou, Angew. Chem., Int. Ed., 2015, 54, 5112 CrossRef CAS PubMed.
- (a) B. Loges, A. Boddien, F. Gärtner, H. Junge and M. Beller, Top. Catal., 2010, 53, 902 CrossRef CAS; (b) A. Boddien, F. Gärtner, C. Federsel, P. Sponholz, D. Mellmann, R. Jackstell, H. Junge and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 6411 CrossRef CAS PubMed; (c) M. Grasemann and G. Laurenczy, Energy Environ. Sci., 2012, 5, 8171 RSC; (d) A. F. Dalebrook, W. Gan, M. Grasemann, S. Moret and G. Laurenczy, Chem. Commun., 2013, 49, 8735 RSC.
- (a) B. Loges, A. Boddien, H. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 3962 CrossRef CAS PubMed; (b) T. C. Johnson, D. J. Morris and M. Wills, Chem. Soc. Rev., 2010, 39, 81 RSC; (c) A. Boddien, D. Mellmann, F. Gärtner, R. Jackstell, H. Junge, P. J. Dyson, G. Laurenczy, R. Ludwig and M. Beller, Science, 2011, 333, 1733 CrossRef CAS PubMed; (d) J. F. Hull, Y. Himeda, W.-H. Wang, B. Hashiguchi, R. Periana, D. J. Szalda, J. T. Muckerman and E. Fujita, Nat. Chem., 2012, 4, 383 CrossRef CAS PubMed; (e) Y. Maenaka, T. Suenobu and S. Fukuzumi, Energy Environ. Sci., 2012, 5, 7360 RSC; (f) J. H. Barnard, C. Wang, N. G. Berry and J. Xiao, Chem. Sci., 2013, 4, 1234 RSC; (g) E. A. Bielinski, P. O. Lagaditis, Y. Zhang, B. Q. Mercado, C. Würtele, W. H. Bernskoetter, N. Hazari and S. Schneider, J. Am. Chem. Soc., 2014, 136, 10234 CrossRef CAS PubMed.
- (a) R. Noyori and S. Hashiguchi, Acc. Chem. Res., 1997, 30, 97 CrossRef CAS; (b) R. Kadyrov and T. H. Riermeier, Angew. Chem., Int. Ed., 2003, 42, 5472 CrossRef CAS PubMed; (c) T. Ikariya and A. J. Blacker, Acc. Chem. Res., 2007, 40, 1300 CrossRef CAS PubMed; (d) C. Wang, C. Li, X. Wu, A. Pettman and J. Xiao, Angew. Chem., Int. Ed., 2009, 48, 6524 CrossRef CAS PubMed; (e) J.-i. Ito and H. Nishiyama, Tetrahedron Lett., 2014, 55, 3133 CrossRef PubMed.
- (a) A. Togni, C. Breutel, A. Schnyder, F. Spindler, H. Landert and A. Tijani, J. Am. Chem. Soc., 1994, 116, 4062 CrossRef CAS; (b) T. Imamoto, J. Watanabe, Y. Wada, H. Masuda, H. Yamada, H. Tsuruta, S. Matsukawa and K. Yamaguchi, J. Am. Chem. Soc., 1998, 120, 1635 CrossRef CAS; (c) D. Liu, W. Tang and X. Zhang, Org. Lett., 2004, 6, 513 CrossRef CAS PubMed; (d) T. Imamoto, K. Tamura, Z. Zhang, Y. Horiuchi, M. Sugiya, K. Yoshida, A. Yanagisawa and I. D. Gridnev, J. Am. Chem. Soc., 2011, 134, 1754 CrossRef PubMed.
- W. Tang, W. Wang, Y. Chi and X. Zhang, Angew. Chem., Int. Ed., 2003, 42, 3509 CrossRef CAS PubMed.
- Y. Tsuchiya, Y. Hamashima and M. Sodeoka, Org. Lett., 2006, 8, 4851 CrossRef CAS PubMed.
- Examples: (a) J. M. Brown and P. A. Chaloner, J. Chem. Soc., Chem. Commun., 1980, 344 RSC; (b) A. S. C. Chan, J. J. Pluth and J. Halpern, J. Am. Chem. Soc., 1980, 102, 5952 CrossRef CAS; (c) R. Giernoth, H. Heinrich, N. J. Adams, R. J. Deeth, J. Bargon and J. M. Brown, J. Am. Chem. Soc., 2000, 122, 12381 CrossRef CAS; (d) I. D. Gridnev, N. Higashi, K. Asakura and T. Imamoto, J. Am. Chem. Soc., 2000, 122, 7183 CrossRef CAS; (e) M. Kitamura, M. Tsukamoto, Y. Bessho, M. Yoshimura, U. Kobs, M. Widhalm and R. Noyori, J. Am. Chem. Soc., 2002, 124, 6649 CrossRef CAS PubMed.
- B. M. Trost, Chem. – Eur. J., 1998, 4, 2405 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of isolated products. See DOI: 10.1039/c5cc01632k |
| This journal is © The Royal Society of Chemistry 2015 |