Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel microfluidic approach for extremely fast and efficient photochemical transformations in fluoropolymer microcapillary films†
N. M.
Reis
* and
G.
Li Puma
*
Environmental Nanocatalysis & Photoreaction Engineering, Department of Chemical Engineering, Loughborough University, Leicestershire, LE11 3TU, UK. E-mail: n.m.reis@lboro.ac.uk; g.lipuma@lboro.ac.uk
First published on 19th March 2015
Abstract
The unique optical properties of the fluoropolymer microcapillary film (MCF) material combined with the extremely fast photoinactivation of Herpes HSV-1 virus, and photodegradation of indigo carmine, diclofenac and benzoylecgonine in the MCF array photoreactor, demonstrate a new, flexible and inexpensive platform for rapid photochemical transformations, high-throughput process analytics and photochemical synthesis.
Photochemical transformation, including the treatment of fluids with ultraviolet light especially of wavelength lower than 280 nm (UVC), has been in practice for many decades, with the first publication reported by NIH in 1949.1 Since then, several studies have shown that UVC is very effective in e.g. the inactivation of bacterial cells and virus particles,2 the photodegradation of pharmaceuticals and other contaminants of concern,3 and the photochemical synthesis of chemical species.4 However, the range of commercial products and applications to be found at the current time based on photochemical transformations is very limited. We believe that this is due to limited understanding of photoreactor design, unclear scale-up methods, the optical limitation of the reactor materials and the very short light penetration depth in opaque fluids. The reported photoreactor designs vary from e.g. thin-film or falling film reactors, bubble columns with concentric lamps and tubular reactors3,5 based on large-bore (>5 mm in diameter) glass or to plastic tubing. In all cases, the radiation field across the fluid is highly heterogeneous and the region with intense light irradiance is usually limited to a very thin region close to the transparent wall of the photoreactor.6 To mitigate this effect, current design requires methods for promoting significant radial mixing e.g. by promoting turbulence using mixers7 or Dean vortices.8 Consequently, current photoreaction systems are intrinsically flawed since fluid-dynamics and radiation field are highly heterogeneous and photochemical processes are difficult to predict and to scale-up.
In this study, we present a novel microfluidic system for controlled, extremely fast and efficient photochemical transformation of chemical and biological species. The microcapillary film (MCF) photoreaction system (Fig. 1) is made of a flat fluoropolymer ribbon containing an array of microcapillaries running along it, usually in the range of 10 μm up to 1 mm internal diameter, which are irradiated by a source of light. The MCF was produced by Lamina Dielectrics Ltd (Billingshurst, West Sussex, UK) from Teflon® FEP (Dupont, USA) using a novel melt-extrusion process.9
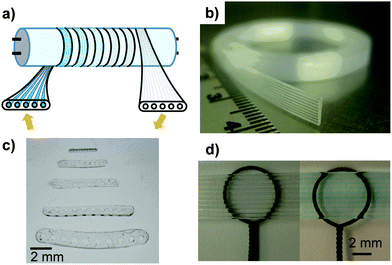 | ||
| Fig. 1 Overview of fluoropolymer MCF microfluidic technology. (a) One possible setup for use in photodegradation of fluids using a tubular lamp and a small bore MCF material wrapped around the bulb. (b) Flexible MCF, showing a parallel array of microcapillaries embedded in a continuous flat plastic film. (c) Microphotograph of the cross-section of 10 bore MCFs produced from Teflon® FEP, having a range of mean hydraulic diameters from 103 ± 7 μm (top) to 494 ± 7 μm (bottom). (d) Outstanding optical transparency of fluoropolymer MCF.10 The left hand image shows capillaries filled with air, with the background image optically distorted as a result of the significant difference in refractive index between air and the wall material. The right image show capillaries filled with a blue dye dissolved in water and no image distortion. | ||
Six unique properties are exemplified in the MCF: (i) the MCF is fully transparent to visible and UV radiation, including UVC; (ii) the flat surface of the film (Fig. 1b) combined with the excellent optical transparency and low refractive index of fluoropolymers (Fig. 1d) allows the irradiation of the entire volume of the fluid flowing in the microcapillaries with straight incident photon rays, without wall refraction effects (such effect is not realized in glass/quartz tubular photoreactors); (iii) the small capillary diameters permit operation at low optical thicknesses11 (less than 0.1) with uniform irradiance of the flowing fluid (even for fluids that in conventional reactors appear to be opaque) and the capillary diameters can be fine-tuned to the incident light and its penetration depth (Fig. 1c); (iv) fluid behaviour approaches the plug flow regime allowing a tight fluid residence time distribution at the reactor exit, and in consequence (in conjunction with (iii)), high reactant conversions, high product yield and high selectivity; (v) the small capillary volumes results in extremely rapid photo-transformations of substrates which are complete in a matter of seconds, a result until now not attainable in other practical photoreactors; (vi) the flexible nature of the MCF makes it easily scalable. We demonstrate these capabilities with a range of applications, including the fast photoinactivation of a highly contagious enveloped Herpes HSV-1 virus particles, the fast decolourization of a dye (indigo carmine) and the photodegradation of contaminants of emerging concern including a pharmaceutical compound (diclofenac) and a class I drug metabolite (benzoylecgonine, BLG). Microstructured reactors and microphotoreactors have been shown in the literature to perform photochemical transformations and to outperform classical photoreactor designs,12 but none of them could accomplish collectively the six unique properties of the MCF photoreaction system described here.
We have measured the fluid behaviour in a 10 bore Teflon® FEP MCF with different mean hydraulic diameters (dh) as shown in Fig. 2a and b (materials and methods are detailed in the ESI†). The residence time distributions were performed by injecting a step of 1000 mg l−1 Blue Dextran 70 at a flow rate of 0.5 ml min−1, and measuring in real-time the absorbance in the whole set of 10 microcapillaries using an Actipix UV microfluidic monitor (Paraytec, York, UK) equipped with a 214 nm dichroic filter. The measured cumulative F-curves were remarkably sharp at a high superficial flow velocity of umean = 42.0–100.0 mm s−1 for the whole array of microcapillaries in MCFs with distinct dh. The dimensionless axial dispersion coefficient, uL/D, estimated by fitting the experimental data to plug flow with the axial dispersion model with open-open boundaries13 ranged from 13.1 to 136.4 for a MCF with dh = 103 μm, and between 14.0 and 42.0 for the larger bore MCF with dh = 159 μm as summarized in Table 1.
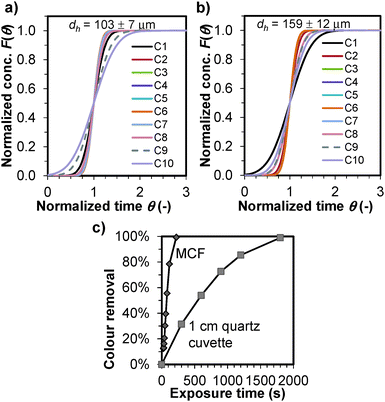 | ||
| Fig. 2 Fluid hydrodynamics and colour removal in the MCF. In (a) and (b) the radial molecular diffusion distance in the MCF is very short, therefore the fluid reveals essentially plug flow behaviour as shown by the large normalized axial dispersion numbers, uL/D summarized in Table 1 for (a) a 10 bore MCF with dh = 103 ± 7 μm and (b) a 10 bore MCF with dh = 159 ± 12 μm. (c) The small internal diameter of the microcapilllaries allows us to fully irradiate all fluid streamlines in the whole volume of the fluid, consequently the UVC photobleaching in a 10 bore MCF with dh = 192 ± 12 μm yields a 10-fold increase in the rate of removal of 20 mg ml−1 indigo carmine (k = 0.0155 s−1, R2 = 0.9777) compared to a 1 cm thick quartz cuvette (k = 0.0016 s−1, R2 = 0.9879) based on a lamp irradiance of 17.9 mW cm−2 for both MCF and quartz cuvette. The exposure time for the MCF in (c) was adjusted by varying the flow rate between 0.2 and 2.0 ml min−1. | ||
| Capillary | uL/D | |
|---|---|---|
| d h = 103 ± 7 μm | d h = 159 ± 12 μm | |
| C1 | 51.8 | 14.0 |
| C2 | 84.7 | 34.4 |
| C3 | 130.2 | 40.4 |
| C4 | 136.4 | 40.4 |
| C5 | 134.1 | 37.3 |
| C6 | 134.1 | 38.9 |
| C7 | 122.3 | 42.0 |
| C8 | 80.4 | 41.8 |
| C9 | 22.6 | 38.4 |
| C10 | 13.1 | 25.7 |
In general, the flow behaviour approached that typical for a plug flow reactor, with maximum values of uL/D about 3.2-fold larger in the smaller bore MCF. This is linked to the shorter time for radial diffusion in smaller diameter capillaries,14 being proportional to the square of the distance according to the Stokes–Einstein equation for molecular diffusion.15 The square of the dh ratios between the two MCFs is ∼3.4, therefore similar to the increase in uL/D, which reveals the importance of using small diameter capillaries with respect to achieving consistent fluid flow performance. The broad distribution in uL/D values in particular at the edge capillaries is linked to the small variation in dh for each microcapillary across the film; with the pressure drop being proportional to dh4 small variations in dh result in significant variations in flow distribution and mean residence time of the liquid across the whole set of microcapillaries. This effect can be minimized by improving the melt-extrusion process.
The UVC photobleaching of indigo carmine dye in the MCF was 8.1-fold faster than in a 1 cm width quartz cuvette (Fig. 2c), although the incident photon flux was identical (17.9 mW cm−2) in both reactors. A linear plot of ln(C/C0) versus time showed a 10-fold increase in the first-order kinetic constant, k. The absorption coefficient at 254 nm of the 20 mg l−1 indigo carmine aqueous solution was 42.5 cm−1, yielding a light penetration distance of 47 μm for 1 log10 unit (i.e. 90%) reduction in transmitted UV-light through the solution. Consequently, in the 1 cm width cuvette a large fraction of the fluid volume was not irradiated at one time, and the volumetric reaction rate dramatically decreased. This is a common occurrence in conventional photoreactors. In the MCF with the small diameter capillaries the entire volume is successfully irradiated and the photodegradation reaction rate significantly increased.
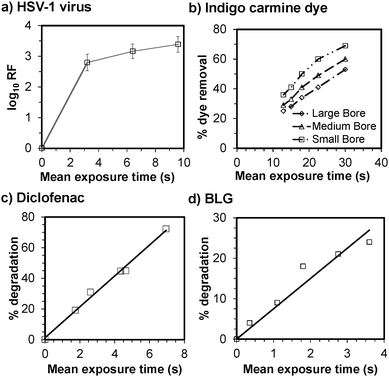
We further applied the MCF to demonstrate the extremely fast photodegradation of a range of molecules and inactivation of biological materials. Firstly, we spiked 10% fetal calf serum (FCS) with ∼107 PFUs of highly infective Herpes virus, HSV-1, and infused the infected opaque solution (Abs254 = 13.9 cm−1) through a 10 bore MCF. A 3 seconds exposure to UVC from a 5 W PL-S5 TUC compact 253.7 nm germicidal lamp (Lamp Specs, Tolworth, UK), arranged longitudinally parallel to the MCF, resulted in ∼3 log10 units reduction factor (RF) in virus titre, and a maximum of 3.4 log10 units with less than 10 s exposure, corresponding to 99.96% of virus particles effectively inactivated. The plateau in data in Fig. 3a is linked to the tailing effect associated with aggregation or resistant virus subpopulations.16
We further investigated the kinetics of UVC bleaching of indigo carmine at a high concentration (100 mg l−1, Abs254 = 212.6 cm−1) with MCFs of different bore diameters wrapped around a 8 W 253.7 nm tubular germicidal lamp (Germicidal G8T5, GE Lighting, Northampton, UK) (Fig. 1a) and found that MCF with smaller dh outperformed those with larger dh (Fig. 3b). In these experiments, the internal volume of the different MCFs shown in Fig. 3b was kept constant at 0.15 ml, by fixing the different exposed length, L, to 1800, 756 and 518 mm for the large, medium and small bore MCFs, respectively. This is associated with more effective light penetration in smaller bore microcapillaries but also to sharper residence time distribution and smaller reactor volumes.
An area of increasing concern is water quality17 and in consequence we investigated the photodegradation of selected contaminants of emerging concern including diclofenac (a pharmaceutical, Fig. 3c) and BLG (the main phototransformation product of cocaine in water, Fig. 3d). As a result of the microfluidic approach adopted here, these compounds degraded in a matter of seconds, which is unprecedented in the literature. By increasing the H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BLG molar ratios up to 700, BLG conversions up to 95% were recorded within a mean exposure time of less than 2.5 s (results not shown). Complete degradation of BLG can also be obtained at smaller H2O2
BLG molar ratios up to 700, BLG conversions up to 95% were recorded within a mean exposure time of less than 2.5 s (results not shown). Complete degradation of BLG can also be obtained at smaller H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BLG molar ratios by increasing the reactor length, although the pressure drop increase would need to be considered. The photodegradation processes shown in Fig. 3b–d revealed a first order kinetics, in theory typical of photochemical processes, however in practice often difficult to measure or obtain with current photoreactor designs because of the intrinsic dependence of reaction kinetics with secondary mixing, radiation field and fluid-dynamics. The inherent flexibility of the MCF material is ideal for fitting tubular or other light sources on a range of scales or for direct exposure to sources of radiation (e.g., sunlight).
BLG molar ratios by increasing the reactor length, although the pressure drop increase would need to be considered. The photodegradation processes shown in Fig. 3b–d revealed a first order kinetics, in theory typical of photochemical processes, however in practice often difficult to measure or obtain with current photoreactor designs because of the intrinsic dependence of reaction kinetics with secondary mixing, radiation field and fluid-dynamics. The inherent flexibility of the MCF material is ideal for fitting tubular or other light sources on a range of scales or for direct exposure to sources of radiation (e.g., sunlight).
We believe that this disruptive and inexpensive microreaction platform technology will provide a new breakthrough in the fields of analytical chemistry, photochemistry and photobiology since it minimizes the intrinsic volumetric heterogeneity of the radiation and fluid-dynamics fields encountered in conventional photoreaction systems, therefore allowing a higher degree of control of photochemical transformations. The MCF photoreactor opens new frontiers for studying phototransformations of emerging contaminants and controlled substances,18 identifying transformation by-products, for the photochemical synthesis of chemical species,4 and for designing photochemical units or disposable modules for downstream processing of biopharmaceuticals and plasma derived products. This is particularly relevant for studying phototransformations (kinetics, reaction mechanisms, quantum yields) from very small volumes of highly-priced chemical compounds, metabolites or intermediates, which are currently unrealizable using other photo-irradiation systems. The liquid volumes used in the MCF are of the order of fractions of ml. This combined with improved reaction efficiency, product yield and selectivity results in reduced treatment cost, increased speed and safety.
The MCF photoreactor can also be easily scaled-up to suit the typical throughput of high value added biomanufacturing products, by increasing the internal diameter (Fig. 1c) and/or the number of microcapillaries. The photosynthesis, photodegradation and photoinactivation processes become fully scalable as the reaction kinetics of the photoprocess is decoupled from the radiation field (often invariant across the capillary cross section at optical thicknesses < 0.1, which are easily attainable in the MCF) and fluid-dynamics. The light penetration and fluid flow through the plastic MCF is fully predicted and governed by well-defined radiation and fluid-dynamics laws, in particular the flow is laminar, therefore the fluid velocity profile is parabolic, and the radiation field (irradiance) is fully described by the Lambert–Beer law. In addition, with small diameter microcapillaries, typically 1 mm or less and preferably 500 μm or less, molecular diffusion becomes significant further promoting the radial mixing of the fluid resulting in a narrow range of residence time distributions across the fluid streamlines. Collectively, the above factors contribute to delivering superior photoreaction transformation products or efficient contaminant removal, which exceeds the specifications of any other photoreactor design.
In summary, the unique optical properties of the fluoropolymer MCF material combined with the extremely fast inactivation of HSV-1 virus particles, and photodegradation of the dye, diclofenac and BLG herein reported in the MCF array photoreactor provides a breakthrough to explore the advantages of non-invasive and efficient phototransformations of uncommon and regulated substances in a simple, flexible and scalable microreactor format, with multiple applications from small scale kinetic characterization to full scale manufacturing using a single-affordable and semi-disposable microfluidic platform. For large-scale applications we envisage in the near future extruding wider MCF films containing hundreds or thousands of microcapillaries embedded in a single fluoropolymer plastic sheet. When the MCF is coupled with advanced analytical mass spectrometry techniques, new frontiers in photochemistry and environmental science can be explored.
The authors thank Dr Colin Crump and Dr Yudan Ren from Department of Biochemistry, Cambridge University, UK; Rosemary Prece, Kashaf Khalid and Abdullahi Geedi from Loughborough University, UK; Aydın Cihanoğlu from Ege University, Turkey; Marianna Vaccaro and Danilo Spasiano from Università degli Studi di Napoli Federico II, Italy, for help in collecting the experimental data shown in the manuscript; Patrick Hester from Lamina Dielectrics Ltd for donating the MCF material, also Professor Malcolm Mackley from the University of Cambridge, UK, for his enthusiasm and support.
Notes and references
- National Institutes of Health, Minimum Requirements: Normal Human Plasma (revision 6), Bethesda, 1949 Search PubMed.
- P. Caillet-Fauquet, M. D. Giambattista, M. L. Draps, F. Sandras, T. Branckaert, Y. D. Launoit and R. Laub, J. Virol. Methods, 2004, 118, 131 CrossRef CAS PubMed; R. F. Dantas, O. Rossiter, A. K. R. Teixeira, A. S. M. Simões and V. L. da Silva, Chem. Eng. J., 2010, 158, 143 CrossRef PubMed.
- (a) R. F. Dantas, O. Rossiter, A. K. R. Teixeira, A. S. M. Simões and V. L. da Silva, Chem. Eng. J., 2010, 158, 143 CrossRef CAS PubMed; (b) N. De la Cruz, J. Giménez, S. Esplugas, D. Grandjean, L. F. De Alencastro and C. Pulgarín, Water Res., 2012, 46, 1947 CrossRef CAS PubMed.
- (a) R. Chong, J. Li, X. Zhou, Y. Ma, J. Yang, L. Huang, H. Han, F. Zhang and C. Li, Chem. Commun., 2014, 50, 165 RSC; (b) N. N. B. Kumar, O. A. Mukhina and A. G. Kutateladze, J. Am. Chem. Soc., 2013, 135, 9608 CrossRef CAS PubMed; (c) L. Li, A. Lv, X.-X. Deng, F.-S. Du and Z.-C. Li, Chem. Commun., 2013, 49, 8549 RSC; (d) T. Aotake, H. Tanimoto, H. Hotta, D. Kuzuhara, T. Okujima, H. Uno and H. Yamada, Chem. Commun., 2013, 49, 3661 RSC; (e) L. Palmisano, V. Augugliaro, M. Bellardita, A. Di Paola, E. García López, V. Loddo, G. Marcì, G. Palmisano and S. Yurdakal, ChemSusChem, 2011, 4, 1431 CrossRef CAS PubMed; (f) Y. Shao, C. Yang, W. Gui, Y. Liu and W. Xia, Chem. Commun., 2012, 48, 3560 RSC; (g) C. Wang, W. Zhang, S. Lu, J. Wu and Z. Shi, Chem. Commun., 2008, 5176 RSC.
- (a) G. L. Puma and P. L. Yue, Environ. Sci. Technol., 1999, 33, 3210 CrossRef CAS; (b) S. Souzanchi, F. Vahabzadeh, S. Fazel and S. N. Hosseini, Chem. Eng. J., 2013, 223, 268 CrossRef CAS.
- A. E. Cassano, C. A. Martin, R. J. Brandi and O. M. Alfano, Ind. Eng. Chem. Res., 1996, 34, 2155 CrossRef.
- Q. Li, S. MacDonald, C. Bienek, P. R. Foster and A. J. MacLeod, Biologicals, 2005, 33, 101 CrossRef CAS PubMed.
- J. Wang, A. Mauser, S. F. Chao, K. Remington, R. Treckmann, K. Kaiser, D. Pifat and J. Hotta, Vox Sang., 2004, 86, 230 CrossRef CAS PubMed.
- B. Hallmark, M. R. Mackley and F. Gadala-Maria, Adv. Eng. Mater., 2005, 7, 545 CrossRef CAS PubMed.
- K. N. N. Reis, A. Edwards, N. Slater and M. Mackley, Immunoassays, Methods for Carrying out Immunoassays, Immunoassay Kits and Method for Manufacturing Immunoassay Kits, WO/2011/117579, 2011 Search PubMed.
- J. Colina-Márquez, F. Machuca-Martínez and G. Li Puma, Environ. Sci. Technol., 2010, 44, 5112 CrossRef PubMed.
- (a) K. N. Loponov, J. Lopes, M. Barlog, E. V. Astrova, A. V. Malkov and A. A. Lapkin, Org. Process Res. Dev., 2014, 18, 1443 CrossRef CAS; (b) Y. Su, N. J. W. Straathof, V. Hessel and T. Noël, Chem. – Eur. J., 2014, 20, 10562 CrossRef CAS PubMed; (c) B. Ramos, S. Ookawara, Y. Matsushita and S. Yoshikawa, J. Chem. Eng. Jpn., 2014, 47, 136 CrossRef CAS; (d) K. S. Elvira, R. C. R. Wootton, N. M. Reis, M. R. Mackley and A. J. de Mello, ACS Sustainable Chem. Eng., 2013, 1, 209 CrossRef CAS; (e) M. Oelgemoeller, Chem. Eng. Technol., 2012, 35, 1144 CrossRef PubMed; (f) M. Oelgemöller and O. Shvydkiv, Molecules, 2011, 16, 7522 CrossRef PubMed; (g) B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and T. D. McQuade, Chem. Rev., 2007, 107, 2300 CrossRef CAS PubMed; (h) F.-F. Lü, L.-Z. Wu, L.-P. Zhang, C.-H. Tung and L.-Q. Zheng, Chin. J. Org. Chem., 2006, 26, 599 Search PubMed; (i) K. Jähnisch and U. Dingerdissen, Chem. Eng. Technol., 2005, 28, 426 CrossRef PubMed; (j) H. Ehrich, D. Linke, K. Morgenschweis, M. Baerns and K. Jähnisch, Chimia, 2002, 56, 647 CrossRef CAS; (k) R. C. R. Wootton, R. Fortt and A. J. de Mello, Org. Process Res. Dev., 2002, 6, 187 CrossRef CAS; (l) H. Lu, M. A. Schmidt and K. F. Jensen, Lab Chip, 2001, 1, 22 RSC; (m) C.-H. Tung, L.-Z. Wu, L.-P. Zhang, H.-R. Li, X.-Y. Yi, K. Song, M. Xu, Z.-Y. Yuan, J.-Q. Guan, H.-W. Wang, Y.-M. Ying and X.-H. Xu, Pure Appl. Chem., 2000, 72, 2289 CrossRef CAS.
- O. Levenspiel, Chemical Reaction Engineering, Wiley, New York, 1999 Search PubMed.
- (a) T. Rodrigues, P. Schneider and G. Schneider, Angew. Chem., Int. Ed., 2014, 53, 5750 CrossRef CAS PubMed; (b) R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502 CrossRef CAS PubMed.
- A. Einstein, Ann. Phys., 1905, 322, 549 CrossRef PubMed.
- M. J. Mattle and T. Kohn, Environ. Sci. Technol., 2012, 46, 10022 CrossRef CAS.
- (a) C. Postigo and S. D. Richardson, J. Hazard. Mater., 2014, 279, 461 CrossRef CAS PubMed; (b) S. W. Krasner, H. S. Weinberg, S. D. Richardson, S. J. Pastor, R. Chinn, M. J. Sclimenti, G. D. Onstad and A. D. Thruston Jr., Environ. Sci. Technol., 2006, 40, 7175 CrossRef CAS; (c) W. Jiang, L. Chen, S. R. Batchu, P. R. Gardinali, L. Jasa, B. Marsalek, R. Zboril, D. D. Dionysiou, K. E. O'Shea and V. K. Sharma, Environ. Sci. Technol., 2014, 48, 12164 CrossRef PubMed.
- S. D. Richardson and T. A. Ternes, Anal. Chem., 2014, 86, 2813 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods. See DOI: 10.1039/c5cc01559f |
| This journal is © The Royal Society of Chemistry 2015 |