Cascade upgrading of γ-valerolactone to biofuels†
Kai
Yan
*ab,
Todd
Lafleur
c,
Xu
Wu
b,
Jiajue
Chai
a,
Guosheng
Wu
c and
Xianmei
Xie
*b
aSchool of Engineering, Brown University, Providence, RI 02906, USA. E-mail: kai_yan@brown.edu; xxmsxty@sina.com
bCollege of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 23001, China
cDepartment of Chemistry, Lakehead University, Thunder Bay, ON P7B 5E1, Canada
First published on 12th March 2015
Abstract
Cascade upgrading of γ-valerolactone (GVL), produced from renewable cellulosic biomass, with selective conversion to biofuels pentyl valerate (PV) and pentane in one pot using a bifunctional Pd/HY catalyst is described. Excellent catalytic performance (over 99% conversion of GVL, 60.6% yield of PV and 22.9% yield of pentane) was achieved in one step. These biofuels can be targeted for gasoline and jet fuel applications.
Sustainable production of biofuels from renewable biomass has been demonstrated as a promising alternative to reduce our dependence on the dwindling fossil resources.1 As one of the major products of plant biomass, renewable γ-valerolactone (GVL) has been suggested as a platform for the production of biofuels (e.g., valerate esters and alkanes) and fine chemicals.2 GVL exhibits the most typical characteristics of an ideal sustainable green solvent for the production of either energy or carbon-based consumer products.2b,c These biofuels deliver the large volumes needed for the transport sector and are potentially more sustainable. Along the line for the transformation of GVL as shown in Scheme 1, pentyl valerate (PV) has more appropriate polarities, better volatility, and higher ignition properties than current and alternative candidate biofuels (e.g., 2-methyltetrahydrofuran and ethanol).3 These properties make it compatible for either gasoline or diesel applications.3a,4 The synthesis of PV often requires pentanoic acid (PA) and pentanol, while both of them can be produced from GVL (Scheme 1). PA, a member of short-chain straight fatty acids, is widely used as an intermediate in the manufacture of numerous valuable products,5 while pentanol is a widely used as a solvent and a replacement for gasoline.1c,6 Further dehydration of pentanol and subsequent hydrogenation would produce pentane in the line (Scheme 1), wherein pentane is a highly attractive transportation fuel. The efficient and selective synthesis of these biofuels (e.g., PV, pentanol and pentane) in one step without the use of extra alcohol reactant under mild conditions will be ideal for the practical utilization.
Lange et al.3a reported the successful synthesis of valerate biofuels through the integration of several steps and found that GVL could be directly converted into PV (20–50% selectivity) using Pt/TiO2 or Pd/TiO2 catalysts at 275–300 °C in a fixed-bed reactor. Besides, they further demonstrated that 90% yield of PA could be obtained from levulinic acid (LA) using Pt-loaded onto a SiO2-bound H-ZSM-5 catalyst. Through catalyst regeneration by calcination in air, the catalytic activity in the production of PA can be maintained. To reduce the high temperature and improve the yield of PV, Chan-Thaw et al.7 selectively synthesized PV from GVL and pentanol using a Cu/SiO2–ZrO2 catalyst in a batch reactor. PV can be obtained at a conversion of over 90% and selectivity of up to 83% in pentanol at 250 °C and 20 h, where pentanol was used as the reactant and solvent with a GVL versus pentanol molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Several other groups have reported the selective production of PV through the hydrogenation of LA in ethanol or pentanol solvent. Good performances have been reported, wherein ethyl levulinate (EV) and PV were often produced together.5,8 LA is relatively easily hydrogenated to PA in a relatively high yield, which undergoes subsequent esterification with pentanol to produce PV. The above systems are advanced. However, extra alcohol fuels (pentanol or ethanol) must be also used as reactants and solvents, which may not follow with the requirements of sustainable development. Besides, some of the reaction systems are often operated at high temperature. The strong corrosiveness of gas-phase LA inputs special requirements toward the fixtures, which limits the practical utilization. A more promising route is to efficiently produce PV in a high yield without the use of extra alcohol reactant in one step under mild conditions.
10. Several other groups have reported the selective production of PV through the hydrogenation of LA in ethanol or pentanol solvent. Good performances have been reported, wherein ethyl levulinate (EV) and PV were often produced together.5,8 LA is relatively easily hydrogenated to PA in a relatively high yield, which undergoes subsequent esterification with pentanol to produce PV. The above systems are advanced. However, extra alcohol fuels (pentanol or ethanol) must be also used as reactants and solvents, which may not follow with the requirements of sustainable development. Besides, some of the reaction systems are often operated at high temperature. The strong corrosiveness of gas-phase LA inputs special requirements toward the fixtures, which limits the practical utilization. A more promising route is to efficiently produce PV in a high yield without the use of extra alcohol reactant in one step under mild conditions.
So far, the conversion of GVL to liquid hydrocarbon fuels has been reported to follow two possible routes (Scheme S1, ESI†). One route (Scheme S1a, ESI†) is to produce pentanoic acid and further upgrade it to butene, which can be subsequently oligomerized to C12 alkanes.1a,9 In the other route (Scheme S1b, ESI†) the production of PA occurs with subsequent ketonization to 5-nonanone, which can be further upgraded to C9 alkanes or C18 alkanes.10 So far, the one-step production of C5 alkanes from GVL has not been reported. It is highly desirable to develop selective cascade upgrading of GVL for the efficient production of biofuels (PV, pentanol and pentane) in one step without the use of the extra alcohol under mild conditions. Herein, we report a one-step selective and flexible cascade upgrading of GVL with perfect conversion to biofuels (60.6% yield of PV and 22.9% yield of pentane) without the use of additional alcohol reactants in a batch reactor.
Upgrading of GVL to PV theoretically proceeds in four steps: (i) opening of the GVL ring and further dehydration to pentanoic acid; (ii) subsequent hydrogenation of pentanoic acid to PA; (iii) partial hydrogenation of PA to pentanol; (iv) esterification of PA and pentanol to PV. However, the conversion of GVL to pentane precedes the same first three steps (i–iii) and the different step (iv) composed of the dehydration of pentanol and subsequent hydrogenation to pentane. In our case, for the selective production of PV and pentane these four steps occurred in one step using a bifunctional Pd/HY catalyst. From the initial screening of the five different supported Pd catalysts (Fig. S1, ESI†), it was clearly seen that the bifunctional Pd/HY catalyst displayed high selectivity toward the formation of PV and pentane, where the metal Pd site was efficient for the hydrogenation and the acidic sites of the HY support promoted the dehydration and esterification. Besides, there was no significant difference in terms of the activity at higher loadings of Pd. Thus, we further concentrated upon the 5% Pd/HY catalyst for catalytic upgrading of GVL to biofuels (PV and pentane). X-ray diffraction (XRD) patterns (Fig. S2, ESI†) indexed as 2θ of (111) at 40.1°, (200) at 46.3°, (220) at 67.7°, and (311) at 81.8° were identified as a single fcc phase of Pd.11 The diffraction intensity of fcc Pd increased with the metal loading from 3% to 5% (Fig. S2b and c, ESI†). The formation of Pd(0) was further confirmed using X-ray photoelectron spectroscopy (XPS), as shown in Fig. S3 (ESI†). The presence of two prominent sets of Pd (3d) peaks, corresponding to the 3d3/2 (340.6 eV) and 3d5/2 (335.3 eV) orbital states, demonstrated that Pd(0) was present on the surface.12 Besides, very weak peaks were observed at 341.7 eV and 336.5 eV, which was possible due to the interaction between the Pd and the oxygen from the HY support.11b,12a Additional evidence for the existence of each element (Si, Al, and Pd) was provided using energy-dispersive X-ray spectroscopy (EDX) (Fig. S4, ESI†), the peaks appeared where the element should be.
To improve the yields of biofuels (PV and pentane), we further investigated the crucial reaction parameters (Fig. 1). It was found that lower temperature (260 °C), higher pressure (80 bar H2) and longer reaction time (24 h) are better for the production of PV. 50.5% yield of PV and 13.1% yield of pentanol were obtained at 260 °C, 24 h and 80 bar H2. As shown in Fig. 1A, the reaction time and hydrogen pressure have a crucial influence on the yield of PV. Upon prolonging the reaction time to 30 h at higher pressure (80 bar), the yield of PV increased significantly and 60.6% yield of PV was achieved. So far, this is the highest yield of PV reported for heterogeneous catalysts in a batch reactor system. The GC spectrum for this case is shown in Fig. S5a (ESI†). Trace amounts of side products (e.g., 1,1-oxybispentane ether, 1,4-pentanediol and MTHF) were also detected. Further prolonging the reaction time to 36 h, the side reactions were clearly promoted. For practical utilization, both high yields and low costs are required. In our case, the GVL/catalyst weight ratio was 22, which is 2-fold higher than the previously reported value of 10.7
For the selective production of pentane, it was observed that the yield was increased at higher temperature (280 °C) and longer reaction time (24 h), which was due to the step (iii) being enhanced under these conditions as suggested by the increased amount of the pentanol intermediate. The highest yield of pentane of 22.9% was obtained at 280 °C, 24 h and 40 bar H2. To the best of our knowledge, this is the first time that production of pentane from GVL in such a high yield has been reported. The GC spectrum for this case is shown in Fig. S5b (ESI†). Traces of butene and 5-nonanone were not found in the gas product from our GC results. We attributed the formation of small quantities of MTHF and 1,1-oxybispentane ether generated through the deep hydrogenation of GVL and potential dehydration of pentanol to 1,1-oxybispentane ether. At 280 °C, more hydrogen (e.g., 80 bar) was disadvantageous for the selective production of pentane because the side products MTHF, 1,4-pentanediol and 1,1-oxybispentane ether were clearly increased. Besides, it was found that a high amount of PA was produced under the conditions of low temperature (260 °C), low pressure (40 bar H2) and short reaction time (12 h), as shown in Fig. 1A. For comparison, we further reduced the reaction temperature and it was clearly found to have a crucial influence on the yield of PA (Fig. 1B). The highest PA yield (over 73%) was obtained at a low temperature of 240 °C, for 12 h and 40 bar H2. This performance is much higher than that obtained in a batch reactor, as reported in a previous report.5a
To understand the reaction pathway, we tested different intermediates and the catalytic results are compared in Fig. S6 (ESI†). When PA was chosen as the reactant, a low conversion of 37.5% was observed, which was possible due to the strong coke formation when the catalyst was exposed to a pure acidic environment, as previously reported by Luo et al.5a In this case, the main product was a PV ester and 1,1-oxybispentane ether, which suggested that two reactions occurred. One was the esterification of PA and the produced pentanol to the PV ester. The other was the dehydration of pentanol that occurred on acidic sites to 1,1-oxybispentane ether. In the case of pentanol as the reactant, a large amount of ether and a small amount of PV were produced. A small amount of pentane was also traced in this case. When pentanol and PA were selectively chosen as substrates, the PV yield increased clearly to 53.5% in 12 h. Based on these data, the probable reaction pathway is proposed in Scheme S2 (ESI†).
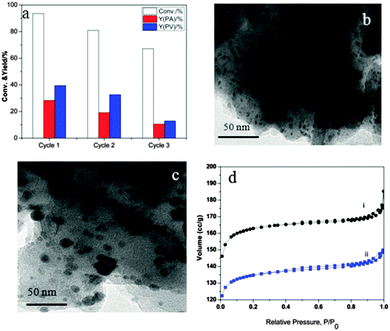
To check the recyclability of the 5% Pd/HY catalyst, recycling experiments were performed under the test conditions 260 °C, 12 h and 80 bar H2. The catalyst can be easily separated from the reaction solution by simple centrifugation. The catalyst was then dried at 90 °C for 12 h and then used for the GVL upgrading. The catalytic results are shown in Fig. 2a. 41.5% yield of PV and 29.3% yield of PA at a conversion of 93.6% GVL were obtained, which were close to the catalytic performance on the fresh 5% Pd/HY catalyst under these conditions. A good performance was possible due to the beneficial H2 and the hydrophobic character of the HY zeolite in octane solvent that reduced the coke formation.14 However, in the second and the third cycles, a decrease in performance was observed. In the third cycle, a poor performance was observed with 12.9% PV and 10.5% PA at 67.2% conversion of GVL, which was possible due to the coke formation or the carbon deposit on the surface over several cycles.3a,14
XRD was used to explore the structural stability of the spent 5% Pd/HY catalyst. As shown in Fig. S7 (ESI†), the resulting metal catalysts (a and b) display similar phase structures, which confirmed that the catalyst was stable under the reaction conditions. Transmission electron microscopy (TEM) images of the fresh 5% Pd/HY (Fig. 2b) and the spent 5% Pd/HY (Fig. 2c) catalyst recycled after the third run, demonstrated no clear crystalline structure change, which is in good agreement with XRD analysis (Fig. S7, ESI†). However, clear aggregation and bigger particle sizes of Pd in the spent 5% Pd/HY catalyst after the third run (Fig. 2c) were observed, which was possible due to exposure of Pd nanoparticles to the reaction environment at 260 °C for long reaction times over several recycles. XPS (Fig. S8, ESI†) was further used to understand the Pd change on the surface and displayed two prominent sets of Pd (3d3/2 and 3d5/2) peaks at 342.8 eV (3d3/2) and 337.1 eV (3d5/2), confirming the visible Pd oxide state in the spent case.11b Temperature-programmed desorption of ammonia (NH3-TPD) analysis (Fig. S9, ESI†) was used to compare the acidity change between the fresh and spent catalysts; it was clearly found that that the peaks have clear shifts, which indicated that the acidic sites were possibly covered.5a,13 Additional evidence was revealed by N2 adsorption–desorption (BET) analysis. The N2-isotherms of the fresh 5% Pd/HY catalyst displays the classic type II curve of microporous materials (Fig. 2d, (i)) and the spent 5% Pd/HY catalyst exhibits the typical IV isotherm of mesoporous structures (Fig. 2d, (ii)). It indicated increased particle sizes and expanded pores in the spent catalyst, which matched well with the above TEM analysis (Fig. 2b and c). The surface areas, pore sizes and the pore volume of the HY support and fresh 5% Pd/HY and spent 5% Pd/HY catalysts are given in Table S1 (ESI†). The details of BET plots and pore size distribution are shown in Fig. S10 (ESI†). In comparison with the HY support (Table S1, no. 1, ESI†), a little larger pore size of 2.12 nm, a lower BET surface area of 451 m2 g−1 with the micropore volume of 0.1850 cm3 g−1 (no. 2) were obtained for the fresh 5% Pd/HY catalyst. It indicated that the Pd nanoparticles were successfully introduced on the porous HY zeolite support. However, in the case of the spent 5% Pd/HY catalyst recycled after the third cycle, the pore size was increased largely to 5 nm, the surface area was reduced to 135 m2 g−1 and the microporous volume was decreased to 0.0201 cm3 g−1 (no. 3). These data clearly confirmed the increased aggregation of metal nanoparticles and more pore blocking over several recycles.
To get rid of the possible carbon deposit or coke on the catalyst surface, simple calcination and further reduction of the spent catalyst was performed (more details are given in the ESI†) and the resulting catalyst was used for GVL upgrading. The TEM image (Fig. 3a) of the reduced 5% Pd/HY catalyst in the fourth run confirmed no structure change. N2-sorption analysis (Fig. 3b) further suggested a stable porous structure as indicated by the similar values of the surface area, pore size as well as pore volume (Table S1, no. 4, ESI†) to those of the fresh 5% Pd/HY catalyst (Table S1, no. 2, ESI†). Thereby, a better distribution with a lower degree of pore-blocking was conceivable. XPS (Fig. 3c) further confirmed the successful reduction and the absence of the oxidation state of PdO. We further employed the reduced catalyst in the catalytic GVL upgrading and stable performance was obtained over several runs (Fig. 3d). Even in the fourth run, 45.7% PV and 41.0% PA were produced at 98.7% conversion of GVL. Analysis of the reaction solution showed that no detectable leaching of Pd was found. The high stability was also possible due to the increased hydrophobic character of the HY zeolite in octane solvent.14
In conclusion, a one-step selective cascade upgrading of GVL to biofuels pentyl valerate and pentane has been successfully achieved using a bifunctional 5% Pd/HY catalyst. 60.6% yield of pentyl valerate and 22.9% yield of pentane were flexibly produced upon the perfect conversion of GVL in a batch reactor system without the use of an additional pentanol reactant and/or a solvent under relatively mild conditions. The bifunctional Pd/HY catalyst maintained stable performance over several runs. The catalytic system and the bifunctional catalyst developed are promising for general biofuel upgrading processes.
K. Yan is thankful for the help from Prof. Dr A. Peterson at Brown University, and the kind introduction to this area by PhD supervisor Prof. Dr W. Leitner (RWTH Aachen University) and Dr N. Theyssen (Max-Planck-Institute for Coal Research). The authors acknowledge the fund from the National Natural Science Foundation of China (Grant No. 50872086) and the Science Technology key projects in Shanxi Province (Grant No. 20130321033-02).
Notes and references
- (a) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS PubMed; (b) S. Crossley, J. Faria, M. Shen and D. E. Resasco, Science, 2010, 327, 68 CrossRef CAS PubMed; (c) J. Yang, N. Li, G. Li, W. Wang, A. Wang, X. Wang, Y. Cong and T. Zhang, Chem. Commun., 2014, 50, 2572 RSC; (d) Q. Liu, N. Wang, J. Caro and A. Huang, J. Am. Chem. Soc., 2013, 135, 17679 CrossRef CAS PubMed; (e) R. Rinaldi, Angew. Chem., Int. Ed., 2014, 53, 8559 CrossRef CAS PubMed.
- (a) J. Q. Bond, D. M. Alonso, D. Wang, R. M. West and J. A. Dumesic, Science, 2010, 327, 1110 CrossRef CAS PubMed; (b) V. Fabos, G. Koczo, H. Mehdi, L. Boda and I. T. Horvath, Energy Environ. Sci., 2009, 2, 767 RSC; (c) I. T. Horvath, H. Mehdi, V. Fabos, L. Boda and L. T. Mika, Green Chem., 2008, 10, 238 RSC.
- (a) J. P. Lange, R. Price, P. M. Ayoub, J. Louis, L. Petrus, L. Clarke and H. Gosselink, Angew. Chem., Int. Ed., 2010, 49, 4479 CrossRef CAS PubMed; (b) F. Geilen, B. Engendahl, A. Harwardt, W. Marquardt, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2010, 49, 5510 CrossRef CAS PubMed; (c) P. Kwanchareon, A. Luengnaruemitchai and S. Jai-In, Fuel, 2007, 86, 1053 CrossRef CAS PubMed.
- (a) J. P. Lange, Biofuels, Bioprod. Biorefin., 2007, 1, 39 CrossRef CAS; (b) T. Pan, J. Deng, Q. Xu, Y. Xu, Q. X. Guo and Y. Fu, Green Chem., 2013, 15, 2967 RSC; (c) R. Palkovits, Angew. Chem., Int. Ed., 2010, 49, 4336 CrossRef CAS PubMed.
- (a) W. Luo, U. Deka, A. M. Beale, E. R. H. van Eck, P. C. A. Bruijnincx and B. M. Weckhuysen, J. Catal., 2013, 301, 175 CrossRef CAS PubMed; (b) P. Sun, G. Gao, Z. Zhao, C. Xia and F. Li, ACS Catal., 2014, 4, 4136 CrossRef CAS; (c) C. Michel, J. Zaffran, A. M. Ruppert, J. Matras-Michalska, M. Jdrzejczyk, J. Grams and P. Sautet, Chem. Commun., 2014, 50, 12450 RSC.
- K. Yan, G. Wu, T. Lafleur and C. Jarvis, Renewable Sustainable Energy Rev., 2014, 38, 663 CrossRef CAS PubMed.
- C. E. Chan-Thaw, M. Marelli, R. Psaro, N. Ravasio and F. Zaccheria, RSC Adv., 2013, 3, 1302 RSC.
- (a) T. Pan, J. Deng, Q. Xu, Y. Xu, Q. X. Guo and Y. Fu, Green Chem., 2013, 15, 2967 RSC; (b) H. N. Pham, Y. J. Pagan-Torres, J. C. Serrano-Ruiz, D. Wang, J. A. Dumesic and A. K. Datye, Appl. Catal., A, 2011, 397, 153 CrossRef CAS PubMed; (c) J. C. Serrano-Ruiz, D. Wang and J. A. Dumesic, Green Chem., 2010, 12, 574 RSC.
- J. Q. Bond, D. Wang, D. M. Alonso and J. A. Dumesic, J. Catal., 2011, 281, 290 CrossRef CAS PubMed.
- (a) J. C. Serrano-Ruiz, D. J. Braden, R. M. West and J. A. Dumesic, Appl. Catal., B, 2010, 100, 184 CrossRef CAS PubMed; (b) J. Q. Bond, D. Martin Alonso, R. M. West and J. A. Dumesic, Langmuir, 2010, 26, 16291 CrossRef CAS PubMed.
- (a) S. Saravanamurugan, M. Paniagua, J. A. Melero and A. Riisager, J. Am. Chem. Soc., 2013, 135, 5246 CrossRef CAS PubMed; (b) W. Fu, L. Zhang, T. Tang, Q. Ke, S. Wang, J. Hu, G. Fang, J. Li and F. S. Xiao, J. Am. Chem. Soc., 2011, 133, 15346 CrossRef CAS PubMed.
- (a) K. Yan, T. Lafleur, G. Wu, J. Liao, C. Ceng and X. Xie, Appl. Catal., A, 2013, 468, 52 CrossRef CAS PubMed; (b) S. J. Tauster, S. C. Fung and R. L. Garten, J. Am. Chem. Soc., 1978, 100, 170 CrossRef CAS.
- W. Luo, P. C. A. Bruijnincx and B. M. Weckhuysen, J. Catal., 2014, 320, 33 CrossRef CAS PubMed.
- P. A. Zapata, J. Faria, M. P. Ruiz, R. E. Jentoft and D. E. Resasco, J. Am. Chem. Soc., 2012, 134, 8570 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization information of XRD, EDX, SEM, XPS and NH3-TPD analyses. See DOI: 10.1039/c5cc01463h |
| This journal is © The Royal Society of Chemistry 2015 |