Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Zincation of 4,4-dimethyloxazoline using TMPZnCl·LiCl. A new preparation of 2-aryloxazolines†
Diana
Haas
,
Maximilian S.
Hofmayer
,
Tomke
Bresser
and
Paul
Knochel
*
Ludwig Maximilians-University Munich, Department of Chemistry, Butenandtstrasse 5-13, 81377 München, Germany. E-mail: paul.knochel@cup.uni-muenchen.de
First published on 13th March 2015
Abstract
The metalation of 4,4-dimethyloxazoline using TMPZnCl·LiCl provides a stable 2-zincated oxazolinyl reagent which readily undergoes palladium-catalyzed Negishi cross-couplings allowing a new access to 2-aryloxazolines. Cu-mediated acylation and allylation reactions also proceed in good yields.
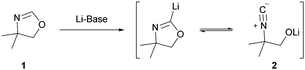
Oxazolines are an important class of heterocycles that display cytotoxic, antitumor, neuroprotective, antibiotic, or antifungal properties,1 but also possess high utility in organic synthesis2 since this heterocycle was found to be an excellent ortho-directing group.3 However, the direct lithiation of oxazolines in position 24 is difficult due to a facile fragmentation of these heterocycles leading to isonitriles of type 2 (Scheme 1).5
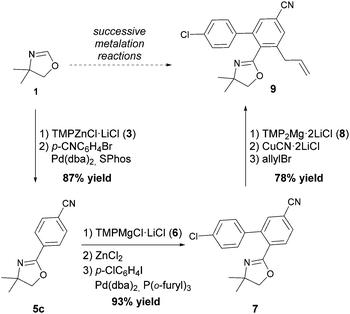
2-Aryloxazolines can be prepared using several methods, such as condensations6 or similar multi-component reactions,7 as well as C–H activations using diverse transition-metal catalysts.8 However, to date the direct zincation and transition-metal catalyzed cross-coupling of the oxazoline scaffold has not yet been reported. This reaction is especially interesting, as 2-aryloxazolines often exhibit potential biological activity.9 Recently, we have described the synthesis of the highly active sterically hindered zinc base TMPZnCl·LiCl10 (3; TMP = 2,2,6,6-tetramethylpiperidyl) which has proven to be especially efficient for the metalation of sensitive heterocycles while displaying an excellent functional group tolerance.11
Herein, we report the direct zincation of 4,4-dimethyloxazoline (1) using TMPZnCl·LiCl (3) and a subsequent quenching of the resulting zinc species with various electrophiles. Thus, treatment of 4,4-dimethyloxazoline (1) with TMPZnCl·LiCl (3, 1.1 equiv.) leads smoothly to the formation of the corresponding 4,4-dimethyloxazolinylzinc (4) species within 1 h at 0 °C in 94% yield as determined by GC-analysis after allylation.12 In contrast to the corresponding lithium derivative (Scheme 1), this zinc reagent is perfectly stable at 25 °C and shows no tendency to undergo a ring fragmentation. Thus, the zinc reagent (4) undergoes smooth carbon–carbon bond formation with electrophiles leading to 2-substituted oxazolines of type 5 in 64–92% yield (Scheme 2).
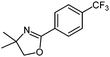
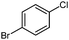
Negishi cross-coupling reactions13 of the oxazolinylzinc reagent (4) with electron-rich and -poor para-functionalized aryl bromides and iodides (FG = CF3, CO2Et, CN, OMe, Cl) using Pd(dba)2 (3 mol%) or Pd(OAc)2 (3 mol%) and SPhos (6 mol%)14 furnished the corresponding 2-aryloxazolines 5a–e in 71–92% yield (Table 1, entries 1–5).
| Entry | Electrophile/conditions | Product/yielda (%) |
|---|---|---|
| a Isolated yield of analytically pure product. b Pd-catalyzed cross-coupling using 3 mol% Pd(OAc)2 and 6 mol% SPhos. c Pd-catalyzed cross-coupling using 3 mol% Pd(dba)2 and 6 mol% SPhos. d A transmetalation with CuCN·2LiCl (1.2 equiv.) was performed. e Pd-catalyzed cross-coupling using 3 mol% Pd(dba)2 and 6 mol% P(o-furyl)3, 4 mol% CuI and NEt3 (1.2 equiv.). | ||
| 1 |

|
|
|
50 °C, 4 h (X = I)
50 °C, 3 h (X = Br) |
5a: 79%b (X = I)
77%c (X = Br) |
|
| 2 |

|

|
|
50 °C, 4 h (X = I)
50 °C, 3 h (X = Br) |
5b: 78%b (X = I)
92%c (X = Br) |
|
| 3 |

|

|
|
50 °C, 4 h (X = I)
50 °C, 2 h (X = Br) |
5c: 80%b (X = I)
87%c (X = Br) |
|
| 4 |

|

|
| 50 °C, 4 h | 5d: 82%b | |
| 5 |
|
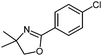
|
| 50 °C, 2 h | 5e: 71%b | |
| 6 |
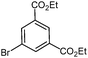
|

|
| 50 °C, 8 h | 5f: 68%c | |
| 7 |

|
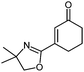
|
| 25 °C, 1 h | 5g: 68%b | |
| 8 |

|

|
| −40 °C to 25 °C, 2 h | 5h: 69%d | |
| 9 |

|
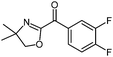
|
| −40 °C to 25 °C, 2 h | 5i: 76%d | |
| 10 |

|
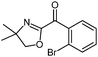
|
| −40 °C to 25 °C, 4 h | 5j: 71%d | |
| 11 |
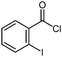
|

|
| −40 °C to 25 °C, 4 h | 5k: 65%d | |
| 12 |

|

|
| −40 °C to 25 °C, 2 h | 5l: 64%d | |
| 13 |

|

|
| −40 °C to 25 °C, 6 h | 5m: 79%d | |
| 14 |

|
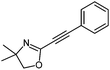
|
| 25 °C, 4 h | 5n: 71%e | |
Also, the oxazolinylzinc species (4) reacts with the disubstituted diethyl 5-bromoisophthalate to provide the desired product (5f) in 68% yield (entry 6). Additionally, a cross-coupling reaction with 3-iodocyclohexenone affords the oxazoline (5g) in 68% yield (entry 7). Moreover, after transmetalation with CuCN·2LiCl15 acylation reactions employing various acid chlorides afford 2-acyl-2-oxazolines.16 Thus, the reaction with benzoyl chloride leads to the acylated oxazoline (5h) in 69% yield (entry 8). The 4,4-dimethyloxazolinylzinc reagent (4) also reacts well with various electron-poor benzoyl chlorides bearing halogen-substituents, as well as 2-thiophenecarbonyl chloride to provide the ketones (5i–l) in 64–76% yield (entries 9–12). In addition, the zincated species undergoes a Cu-mediated allylation reaction with 3-bromocyclohexene affording the allylated product (5m) in 79% yield (entry 13). Finally, an in situ performed Sonogashira reaction17 with phenylacetylene in the presence of Pd(dba)2 (3 mol%), Farina's ligand18 (P(o-furyl)3, 6 mol%), CuI (4 mol%) and Et3N afforded the 2-substituted oxazoline (5n) in 79% yield (entry 14). As oxazolines serve as a useful directing group in directed metalations,19 magnesiation of the 2-arylated oxazolines is possible within 1.5 h at 0 °C using TMPMgCl·LiCl20 (6, Scheme 3).21 After transmetalation with ZnCl2 and Pd-catalyzed cross-coupling, the biaryl 7 is afforded in 93% yield. Also, oxazoline 7 reacts in a second metalation step using TMP2Mg·2LiCl22 (8, −20 °C, 1.5 h). Transmetalation using CuCN·2LiCl, followed by addition of allyl bromide furnishes the desired oxazoline 9 in 78% yield. Since oxazolines can be readily converted to carboxylic acids, new ortho,ortho′-disubstituted benzoic acids can be obtained after deprotection using standard methods.23
In summary, we have reported a simple, mild and efficient method for the zincation of 4,4-dimethyloxazoline in position 2 using TMPZnCl·LiCl (3). The resulting oxazolinylzinc reagents (4) were arylated via Negishi cross-couplings with various aryl iodides and bromides. Additionally, Cu-mediated acylation and allylation reactions proceed readily. Applications towards the synthesis of biologically active molecules are currently being investigated in our laboratories.
We thank the European Research Council under the European Community's Seventh Framework Programme (FP7/2007–2013; ERC grant agreement no. 227763) and the Deutsche Forschungsgemeinschaft (DFG) for financial support. We also thank BASF SE (Ludwigshafen), W. C. Heraeus GmbH (Hanau) and Rockwood Lithium GmbH (Frankfurt) for the generous gift of chemicals.
Notes and references
- (a) S. Yamamoto, N. Okujo, Y. Fujita, M. Saito, T. Yoshida and S. J. Shinoda, J. Biochem., 1993, 113, 538 CAS; (b) H. Shitakawa, S. Nakajima, M. Hirayama, H. Kondo and K. Kojiri, Chem. Abstr., 2000, 132, 150670 Search PubMed; (c) M. R. Prinsep, R. E. Moore, I. A. Levine and G. M. L. Patterson, J. Nat. Prod., 1992, 55, 140 CrossRef CAS.
- (a) M. Reuman and A. I. Meyers, Tetrahedron, 1985, 41, 837 CrossRef CAS; (b) A. I. Meyers and E. D. Mihelich, Angew. Chem., Int. Ed., 1976, 15, 270 CrossRef; (c) J. A. Frump, Chem. Rev., 1971, 71, 483 CrossRef CAS.
- (a) S. Oi, E. Aizawa, Y. Ogino and Y. Inoue, J. Org. Chem., 2005, 70, 3113 CrossRef CAS PubMed; (b) P. Beak and R. A. Brown, J. Org. Chem., 1982, 47, 34 CrossRef CAS; (c) P. Beak, A. Tse, J. Hawkins, C. Chen and S. Mills, Tetrahedron, 1983, 39, 1983 CrossRef CAS; (d) V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS; (e) Y. Zhao and V. Snieckus, J. Am. Chem. Soc., 2014, 136, 11224 CrossRef CAS PubMed; (f) K. Groom, S. M. S. Hussain, J. Morin, C. Nilewski, T. Rantanen and V. Snieckus, Org. Lett., 2014, 16, 2378 CrossRef CAS PubMed.
- L. Degennaro, V. Capriati, C. Carlucci, S. Florio, R. Luisi, I. Nuzzo and C. Cuocci, Tetrahedron, 2009, 65, 8745 CrossRef CAS PubMed.
- (a) A. I. Meyers and E. W. Collington, J. Am. Chem. Soc., 1970, 92, 6676 CrossRef CAS; (b) F. Gerhart and U. Schöllkopf, Tetrahedron Lett., 1968, 9, 6231 CrossRef; (c) S. R. Neal, A. Ellern and A. D. Sadow, J. Organomet. Chem., 2011, 696, 228 CrossRef CAS PubMed.
- (a) G. A. Molander, W. Febo-Ayala and L. Jean-Gérard, Org. Lett., 2009, 11, 3830 CrossRef CAS PubMed; (b) P. Garg, S. Chaudhary and M. D. Milton, J. Org. Chem., 2014, 79, 8668 CrossRef CAS PubMed; (c) I. Mohammadpoor-Baltork, A. R. Khosropour and S. F. Hojati, Synlett, 2005, 2747 CrossRef CAS PubMed; (d) M. Brandstätter, F. Roth and N. W. Luedtke, J. Org. Chem., 2015, 80, 40 CrossRef PubMed.
- (a) X.-F. Wu, H. Neumann, S. Neumann and M. Beller, Chem. – Eur. J., 2012, 18, 13619 CrossRef CAS PubMed; (b) L. Fan, E. Lobkovsky and B. Ganem, Org. Lett., 2007, 9, 2015 CrossRef CAS PubMed.
- (a) S. H. Kim and S. Chang, Org. Lett., 2010, 12, 1868 CrossRef CAS PubMed; (b) L. Ackermann, S. Barfüsser, C. Kornhaass and A. R. Kapdi, Org. Lett., 2011, 13, 3082 CrossRef CAS PubMed; (c) S. H. Wiedemann, R. G. Bergman and J. A. Ellman, Org. Lett., 2004, 6, 1685 CrossRef CAS PubMed.
- For examples, see: (a) V. Padmavathi, K. Mahesh, G. D. Reddy and A. Padmaja, Eur. J. Med. Chem., 2010, 45, 3178 CrossRef CAS PubMed; (b) M. Tsuda, M. Yamakawa, S. Oka, Y. Tanaka, Y. Hoshino, Y. Mikami, A. Sato, H. Fujiwara, Y. Ohizumi and J. Kobayashi, J. Nat. Prod., 2005, 68, 462 CrossRef CAS PubMed; (c) Q. Li, K. W. Woods, A. Claireborne, S. L. Gwanltey, K. J. Barr, G. Liu, L. Gehrke, R. B. Credo, Y. Hua Hui, J. Lee, R. B. Warner, P. Kovar, M. A. Nukkla, N. A. Zielinski, S. K. Tahir, M. Fitzgerald, K. H. Kim, K. Marsh, D. Frost, S.-C. Ng, S. Rosenberg and H. L. Sham, Bioorg. Med. Chem., 2002, 12, 465 CrossRef CAS.
- (a) M. Mosrin and P. Knochel, Org. Lett., 2009, 11, 1837 CrossRef CAS PubMed; (b) S. Duez, A. K. Steib, S. M. Manolikakes and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 7686 CrossRef CAS PubMed; (c) A. Unsinn, M. J. Ford and P. Knochel, Org. Lett., 2013, 15, 1128 CrossRef CAS PubMed; (d) D. Haas, M. Mosrin and P. Knochel, Org. Lett., 2013, 15, 6162 CrossRef CAS PubMed.
- For general reviews on the metalation of aromatics and heterocycles see: (a) M. C. Whisler, S. MacNeil, V. Snieckus and P. Beak, Angew. Chem., Int. Ed., 2004, 43, 2206 CrossRef CAS PubMed; (b) R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., Int. Ed., 2007, 46, 3802 CrossRef CAS PubMed; (c) F. Chevallier and F. Mongin, Chem. Soc. Rev., 2008, 37, 595 RSC; (d) B. Haag, M. Mosrin, H. Ila, V. Malakhov and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 9794 CrossRef CAS PubMed.
- Compare Table 1, entry 13; C14H30 was used as an internal standard.
- (a) E. Negishi, A. O. King and N. Okukado, J. Org. Chem., 1977, 42, 1821 CrossRef CAS; (b) E. Negishi, L. F. Valente and M. Kobayashi, J. Am. Chem. Soc., 1980, 102, 3298 CrossRef CAS; (c) E. Negishi and M. Kobayashi, J. Org. Chem., 1980, 45, 5223 CrossRef; (d) E. Negishi, Acc. Chem. Res., 1982, 15, 340 CrossRef CAS; (e) E. Negishi, Angew. Chem., Int. Ed., 2011, 50, 6738 CrossRef CAS PubMed.
- T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685 CrossRef CAS PubMed.
- P. Knochel, M. C. P. Yeh, S. C. Berk and J. Talbert, J. Org. Chem., 1988, 53, 2390 CrossRef CAS.
- (a) V. Capriati, S. Florio, R. Luisi, V. Russo and A. Salomone, Tetrahedron Lett., 2000, 41, 8835 CrossRef CAS; (b) V. Capriati, S. Florio, R. Luisi, V. Russo and A. Salomone, Tetrahedron Lett., 2000, 41, 8835 CrossRef CAS.
- (a) K. Sonogashira, Y. M. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef; (b) R. Chinchilla and C. Nájera, Chem. Rev., 2007, 107, 874 CrossRef CAS PubMed; (c) H. Doucet and J.-C. Hierso, Angew. Chem., Int. Ed., 2007, 46, 834 CrossRef CAS PubMed; (d) R. Chinchilla and C. Nájera, Chem. Soc. Rev., 2011, 40, 5084 RSC; (e) K. Sonogashira, in Metal-catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J Stang, Wiley-VCH, Weinheim, 1998 Search PubMed.
- (a) V. Farina and B. Krishnan, J. Am. Chem. Soc., 1991, 113, 9585 CrossRef CAS; (b) V. Farina, S. R. Baker, D. A. Benigni and C. Sapino Jr., Tetrahedron Lett., 1988, 29, 5739 CrossRef CAS.
- (a) A. I. Meyers and E. D. Mihelich, J. Org. Chem., 1975, 40, 3158 CrossRef CAS; (b) H. A. Chiong, Q.-N. Pham and O. Daugulis, J. Am. Chem. Soc., 2007, 129, 9879 CrossRef CAS PubMed; (c) X. Chen, J.-J. Li, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 78 CrossRef CAS PubMed; (d) S. T. Chadwick, A. Ramirez, L. Gupta and D. B. Collum, J. Am. Chem. Soc., 2007, 129, 2259 CrossRef CAS PubMed.
- (a) A. Krasovskiy, V. Krasovskaya and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 2958 CrossRef CAS PubMed; (b) W. Lin, O. Baron and P. Knochel, Org. Lett., 2006, 8, 5673 CrossRef CAS PubMed; (c) P. García-Alvarez, D. V. Graham, E. Hevia, A. R. Kennedy, J. Klett, R. E. Mulvey, C. T. O’Hara and S. Weatherstone, Angew. Chem., Int. Ed., 2008, 47, 8079 CrossRef PubMed; (d) R. E. Mulvey, Organometallics, 2006, 25, 1060 CrossRef CAS; (e) R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., Int. Ed., 2007, 46, 3802 CrossRef CAS PubMed.
- (a) J. H. C. Batista, F. M. dos Santos, L. A. Bozzini, R. Vessecchi, A. R. M. Oliveira and G. C. Clososki, Eur. J. Org. Chem., 2015, 967 CrossRef CAS; (b) E. Bellamy, O. Bayh, C. Hoarau, F. Trécourt, G. Quéguiner and F. Marsais, Chem. Commun., 2010, 46, 7043 RSC.
- (a) G. C. Clososki, C. J. Rohbogner and P. Knochel, Angew. Chem., Int. Ed., 2007, 46, 7681 CrossRef CAS PubMed; (b) C. J. Rohbogner, G. C. Clososki and P. Knochel, Angew. Chem., Int. Ed., 2008, 47, 1503 CrossRef CAS PubMed; (c) B. Haag, M. Mosrin, H. Ila, V. Malakhov and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 9794 CrossRef CAS PubMed; (d) A. Unsinn, C. J. Rohbogner and P. Knochel, Adv. Synth. Catal., 2013, 355, 1553 CrossRef CAS.
- (a) P. J. Kocienski, Protecting Groups, Georg Thieme Verlag, Stuttgart, 3rd edn, 2005, pp. 107–110 Search PubMed; (b) K. J. Edgar and C. K. Bradsher, J. Org. Chem., 1982, 47, 1585 CrossRef CAS; (c) J. I. Levin and S. M. Weinreb, Tetrahedron Lett., 1982, 23, 2347 CrossRef CAS; (d) C. A. Boulet and G. A. Poulton, Heterocycles, 1989, 28, 405 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and NMR spectra of all products. See DOI: 10.1039/c5cc01144b |
| This journal is © The Royal Society of Chemistry 2015 |