Open Access Article
Open Access ArticleSynthesis of pyrroloindolines and furoindolines via cascade dearomatization of indole derivatives with carbenium ion†
Chuan
Liu
,
Qin
Yin
,
Li-Xin
Dai
and
Shu-Li
You
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China. E-mail: slyou@sioc.ac.cn; Fax: +86 21-5492-5087
First published on 13th February 2015
Abstract
A highly efficient intermolecular cascade dearomatization of substituted indoles with benzodithiolylium tetrafluoroborate has been developed. This reaction provides a novel strategy to synthesise C3 methyl-substituted pyrroloindolines and furoindolines under mild reaction conditions, the utility of which has been demonstrated by the synthesis of esermethol and physovenine in a highly concise manner.
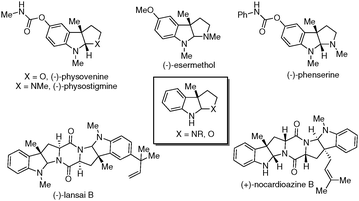
The pyrroloindolines and furoindolines are found as key structural elements in a large number of indole alkaloids1 which exhibit attractive biological activities.2 Within these families, many natural products bearing a C3 methyl group have been identified (Fig. 1). As some of the arrays, analogues of (−)-physostigmine have received substantial interest because of their medicinal properties,3 such as the treatment of myasthenia gravis, glaucoma, delayed gastric emptying and Alzheimer's disease. Therefore, much effort has been made to develop efficient methods to synthesise these molecules.4 Reported methods generally can be categorized into two groups, including the interrupted Fischer indolization reaction and initial synthesis of 3,3-disubstituted oxindoles followed by a subsequent elaboration. Despite the progress being made, the direct synthesis of methyl-substituted pyrroloindolines and furoindolines from substituted indoles is still challenging.
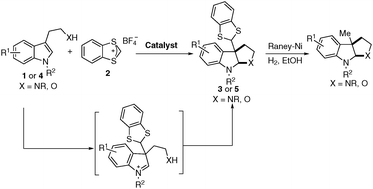
In recent years, cascade dearomatization reactions of indoles5–7 have received considerable attention as they provide efficient and straightforward methods to synthesise [2,3]-fused indoline derivatives. As part of our ongoing research towards the development of dearomatization reactions,8 we envisioned that the C3 methyl-substituted pyrroloindolines and furoindolines could be assembled by the reaction of tryptamines or tryptophols with electrophilic methylation reagents through a cascade electrophilic aromatic addition and iminium ion trapping (Scheme 1). To test this hypothesis, some commonly used methylation reagents (MeI, MeOTf, Me3OBF4 and Me2SO4) have been tested with N1-methyl-N10-carbomethoxytryptamine (1a) under various conditions, however, no desired product was observed. Then we turned our attention to benzodithiolylium tetrafluoroborate (2), which was employed in a formal α-methylation of aldehydes reported by Cozzi's group.9 We found that tryptamines or tryptophols could react with commercially available 2 providing the C3 methyl-substituted pyrroloindolines or furoindolines after a further deprotection of 1,3-benzothiole (Scheme 1).10 Herein, we report such a highly efficient cascade dearomatization of substituted indoles with benzodithiolylium tetrafluoroborate. Furthermore, the synthetic value of this method is well demonstrated by the synthesis of esermethol and physovenine.
In the initial study, N1-methyl-N10-carbomethoxytryptamine (1a)11 and benzodithiolylium tetrafluoroborate (2) were selected as model substrates for the optimization of the reaction conditions.12 In the presence of 10 mol% BINOL-derived phosphoric acid and 110 mol% Na2CO3, the reaction was performed in fluorobenzene at room temperature, and the desired product 3a was obtained in only 20% yield, together with a significant amount of 3aa by further alkylation at the C5-position of indole (entry 1, Table 1). Then we tested the reaction at lower temperature to limit the further alkylation process (entries 2–6, Table 1). Gratifyingly, the yield of 3a could be remarkably enhanced to 89% at −30 °C (entry 5, Table 1). The yield of 3a was further improved to 94% by using toluene as the solvent (entry 7, Table 1). Several commonly used Brønsted acids were investigated (entries 8–10, Table 1) and it was found that the reaction with benzoic acid could provide similar yield (93% yield, entry 10, Table 1). Then benzoic acid was chosen for further screening of the reaction conditions. To our delight, increasing the loading of 1a to 110 mol% resulted in an almost quantitative yield of 3a (99% yield, entry 11, Table 1). It should be noted that only a trace product could be detected in the absence of the base (entries 12 and 13, Table 1). Interestingly, 3a was obtained in 88% yield without the addition of an acid (entry 14, Table 1). The corresponding salt of the acid likely acts as a phase transfer catalyst to promote the alkylation reaction.
| Entry | Acid | Temp. (°C) | Solvent | 3a, Yieldb (%) | 3aa, Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2 (0.11 mmol), Na2CO3 (0.11 mmol) in toluene (1.5 mL) at noted temperature. b Determined by HPLC analysis. c 1a (0.22 mmol), 2 (0.2 mmol), Na2CO3 (0.22 mmol) in toluene (3.0 mL) at −30 °C. d Isolated yield. e Without Na2CO3. f Without acid and Na2CO3. g Without acid. | |||||
| 1 | CPA | rt | C6H5F | 20 | 39 |
| 2 | CPA | 0 | C6H5F | 35 | 37 |
| 3 | CPA | −10 | C6H5F | 74 | 17 |
| 4 | CPA | −20 | C6H5F | 87 | 11 |
| 5 | CPA | −30 | C6H5F | 89 | 4 |
| 6 | CPA | −40 | C6H5F | 68 | 18 |
| 7 | CPA | −30 | Toluene | 94 | 0.5 |
| 8 | CSA | −30 | Toluene | 90 | 6 |
| 9 | TsOH·H2O | −30 | Toluene | 77 | 12 |
| 10 | PhCOOH | −30 | Toluene | 93 | 1.5 |
| 11 | PhCOOH | −30 | Toluene | 99(99 ) | <0.5 |
| 12e | PhCOOH | −30 | Toluene | 3 | 5 |
| 13f | — | −30 | Toluene | 3 | 7 |
| 14g | — | −30 | Toluene | 88 | 5 |
Under the optimized reaction conditions (entry 11, Table 1), the scope of tryptamine derivatives was examined. The results are summarized in Scheme 2. Firstly, substrates with various N1-protected groups on the indole moiety were investigated and it was found that both electron-donating group (methyl, allyl, benzyl) and electron-withdrawing group (CO2Me) were well tolerated, affording the corresponding dearomatized products in 91–99% yields (3a to 3d). Secondly, different protecting groups (Boc, Ts, Me) on the amine of tryptamine were also explored. In the case of Boc and Ts, excellent yields (96–99%, 3e and 3f) were achieved for the desired pyrroloindoline products. However, for N-Me protected tryptamine, only moderate yield was obtained (39%, 3g), due to the nucleophilicity of the amine side chain. Next, varying the position and electronic properties of the substituent (4-Cl, 5-OMe, 5-Me, 5-F, 5-Cl, 5-Br, 6-F, 7-Me) on the indole core could all be tolerated (Scheme 2). In all cases, the pyrroloindoline products were obtained in excellent yields (86–98%, 3h–3o). The structure of products was determined by an X-ray crystallographic analysis of a single crystal of 3f.12
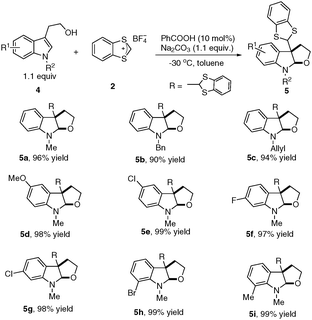
To further broaden the substrate scope, we began to test tryptophols for this cascade dearomatization reaction with carbenium ion (Scheme 3). When N-protected substituted tryptophols were subjected to the optimized conditions, excellent yields (90–96% yields, 5a to 5c) were obtained for the desired furoindoline products. Moreover, examination of the substituent patterns on the indole core revealed that this protocol was compatible with both electron-donating (5-OMe, 7-Me) and electron-withdrawing (5-Cl, 6-F, 6-Cl, 7-Br) group substituted tryptophols.
In addition, a chiral substrate was tested to examine the stereoselective transformation. Tryptophan derivative 6 could be utilized in this cascade dearomatization reaction, providing compound 7 in 97% yield with excellent diastereoselectivity (dr = 94/6, Scheme 4). Further examining the asymmetric version of the current cascade dearomatization reaction only gave the product with a relatively low enantioselectivity (up to 10% ee).12 The low ee value is probably due to the strong background reaction.
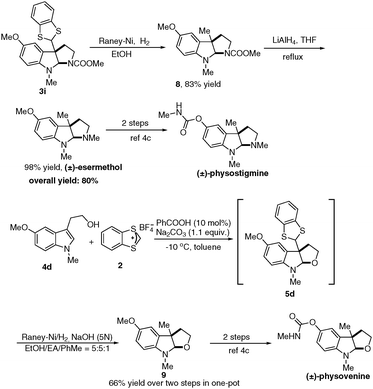
To demonstrate the synthetic value of the current methodology, we applied this method in the synthesis of esermethol and physovenine. As depicted in Scheme 5, under the RANEY® Ni/H2 conditions, deprotection of the 1,3-benzothiole group in 3i easily took place to afford compound 8 in 83% yield. Further reduction of the methylcarbamate moiety with LiAlH4 afforded (±) esermthol in 98% yield. Notably, esermethol is a synthetic precursor of physostigmine and phenserine,4c which are known as acetylcholinesterase inhibitors (Scheme 5). In addition, dearomatization of 4d and subsequent one-pot deprotection of the 1,3-benzothiole group provided compound 9 in 66% yield over two steps. Compound 9 is an intermediate for the synthesis of physovenine.4c
To further demonstrate the utility of the dearomatized products, several transformations have been performed (Scheme 6). Product 3a was easily converted into the corresponding methyl substituted compound 10 after deprotection of the 1,3-benzothiole group. The reduction of 3a with LiAlH4 provided 3g in 68% yield. In addition, methylation product 11 could be obtained by the treatment of 3a with MeI after lithiation by n-BuLi at −30 °C. Furthermore, compound 3c could be converted to 12 in 49% yield after lithiation and subsequent reaction with isobutylene oxide.
In summary, we have developed an efficient method to construct pyrroloindoline and furoindoline skeletons by an intermolecular cascade dearomatization of indole derivatives with carbenium ion. The current reaction features readily available starting materials, wide substrate scope, excellent yields, and mild reaction conditions. The products obtained from this method could undergo diverse transformations including the total synthesis of esermethol and physovenine in a highly concise manner.
We thank the National Basic Research Program of China (973 Program 2015CB856600), National Natural Science Foundation of China (21332009, 21421091), and Chinese Academy of Sciences for generous financial support.
Notes and references
- (a) S. Takano and K. Ogasawara, in The Alkaloids, ed. A. Brossi, Academic, New York, 1989, vol. 36, p. 225 Search PubMed; (b) U. Anthoni, C. Christophersen and P. H. Nielsen, in Alkaloids: Chemical & Biological Perspectives, S. W. Pelletier, ed. Pergamon, Oxford, 1999, vol. 13, p. 163 Search PubMed; (c) T. Suzuki, J.-H. Choi, T. Kawaguchi, K. Yamashita, A. Morita, H. Hirai, K. Nagai, T. Hirose, S. Ōmura, T. Sunazuka and H. Kawagishi, Bioorg. Med. Chem. Lett., 2012, 22, 4246 CrossRef CAS PubMed.
- (a) C.-J. Zheng, C.-J. Kim, K. S. Bae, Y.-H. Kim and W.-G. Kim, J. Nat. Prod., 2006, 69, 1816 CrossRef CAS PubMed; (b) M. Varoglu, T. H. Corbett, F. A. Valeriote and P. Crews, J. Org. Chem., 1997, 62, 7078 CrossRef CAS; (c) Y. Usami, J. Yamaguchi and A. Numata, Heterocycles, 2004, 63, 1123 CrossRef CAS; (d) K.-P. Shaw, Y. Aracava, A. Akaike, J. W. Daly, D. L. Rickett and E. X. Albuquerque, Mol. Pharmacol., 1985, 28, 527 CAS.
- (a) B. Witkop, Heterocycles, 1998, 49, 9 CrossRef CAS PubMed; (b) A. Shafferman, D. Barak, D. Stein, C. Kronman, B. Velan, N. H. Greig and A. Ordentlich, Chem.-Biol. Interact., 2008, 175, 166 CrossRef CAS PubMed.
- For selected examples of synthesis of physostigmine: (a) T. B. K. Lee and G. S. K. Wong, J. Org. Chem., 1991, 56, 872 CrossRef CAS; (b) A. Ashimori, T. Matsuura, L. E. Overman and D. J. Poon, J. Org. Chem., 1993, 58, 6949 CrossRef CAS; (c) T. Matsuura, L. E. Overman and D. J. Poon, J. Am. Chem. Soc., 1998, 120, 6500 CrossRef CAS; (d) M. Kawahra, A. Nishida and M. Nakagawa, Org. Lett., 2000, 2, 675 CrossRef PubMed; (e) A. S. ElAzab, T. Taniguchi and K. Ogasawara, Org. Lett., 2000, 2, 2757 CrossRef CAS PubMed; (f) B. M. Trost and Y. Zhang, J. Am. Chem. Soc., 2006, 128, 4590 CrossRef CAS PubMed; (g) A. W. Schammel, G. Chiou and N. K. Garg, J. Org. Chem., 2012, 77, 725 CrossRef CAS PubMed; (h) T. Araki, T. Ozawa, H. Yokoe, M. Kanematsu, M. Yoshida and K. Shishido, Org. Lett., 2013, 15, 200 CrossRef CAS PubMed.
- For recent reviews on dearomatization reaction of indoles, see: (a) S. P. Roche and J. A. Porco Jr, Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS PubMed; (b) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef PubMed; (c) L. M. Repka and S. E. Reisman, J. Org. Chem., 2013, 78, 12314 CrossRef CAS PubMed.
- For selected organocatalyzed dearomatization of indoles, see: (a) J. F. Austin, S.-G. Kim, C. J. Sinz, W.-J. Xiao and D. W. C. MacMillan, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5482 CrossRef CAS PubMed; (b) C. Gioia, A. Hauville, L. Bernardi, F. Fini and A. Ricci, Angew. Chem., Int. Ed., 2008, 47, 9236 CrossRef CAS PubMed; (c) S. B. Jones, B. Simmons, A. Mastracchio and D. W. C. MacMillan, Nature, 2011, 475, 183 CrossRef CAS PubMed; (d) O. Lozano, G. Blessley, T. M. del Campo, A. L. Thompson, G. T. Giuffredi, M. Bettati, M. Walker, R. Borman and V. Gouverneur, Angew. Chem., Int. Ed., 2011, 50, 8105 CrossRef CAS PubMed; (e) F. Kolundzic, M. N. Noshi, M. Tjandra, M. Movassaghi and S. J. Miller, J. Am. Chem. Soc., 2011, 133, 9104 CrossRef CAS PubMed; (f) B. Tan, G. Hernández-Torres and C. F. Barbas, III, J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS PubMed; (g) Z. Zhang and J. C. Antilla, Angew. Chem., Int. Ed., 2012, 51, 11778 CrossRef CAS PubMed; (h) W. Xie, G. Jiang, H. Liu, J. Hu, X. Pan, H. Zhang, X. Wan, Y. Lai and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 12924 CrossRef CAS PubMed; (i) T. M. Kaiser and J. Yang, Eur. J. Org. Chem., 2013, 3983 CrossRef CAS; (j) H. M. Nelson, S. H. Reisberg, H. P. Shunatona, J. S. Patel and F. D. Toste, Angew. Chem., Int. Ed., 2014, 53, 5600 CrossRef CAS PubMed; (k) L. Liao, C. Shu, M. Zhang, Y. Liao, X. Hu, Y. Zhang, Z. Wu, W. Yuan and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 10471 CrossRef CAS PubMed; (l) C. S. Yeung, R. E. Ziegler, J. A. Porco Jr and E. N. Jacobsen, J. Am. Chem. Soc., 2014, 136, 13614 CrossRef CAS PubMed; (m) Y.-C. Zhang, J.-J. Zhao, F. Jiang, S.-B. Sun and F. Shi, Angew. Chem., Int. Ed., 2014, 53, 13912 CrossRef CAS PubMed.
- For selected metal-catalyzed dearomatization of indoles, see: (a) M. Kimura, M. Futamata, R. Mukai and Y. Tamaru, J. Am. Chem. Soc., 2005, 127, 4592 CrossRef CAS PubMed; (b) B. M. Trost and J. Quancard, J. Am. Chem. Soc., 2006, 128, 6314 CrossRef CAS PubMed; (c) Y. Lian and H. M. L. Davies, J. Am. Chem. Soc., 2010, 132, 440 CrossRef CAS PubMed; (d) L. M. Repka, J. Ni and S. E. Reisman, J. Am. Chem. Soc., 2010, 132, 14418 CrossRef CAS PubMed; (e) N. Shimada, T. Oohara, J. Krishnamurthi, H. Nambu and S. Hashimoto, Org. Lett., 2011, 13, 6284 CrossRef CAS PubMed; (f) S. Zhu and D. W. C. MacMillan, J. Am. Chem. Soc., 2012, 134, 10815 CrossRef CAS PubMed; (g) G. Cera, M. Chiarucci, A. Mazzanti, M. Mancinelli and M. Bandini, Org. Lett., 2012, 14, 1350 CrossRef CAS PubMed; (h) M. E. Kieffer, K. V. Chuang and S. E. Reisman, Chem. Sci., 2012, 3, 3170 RSC; (i) Y. Liu and H. Du, Org. Lett., 2013, 15, 740 CrossRef CAS PubMed; (j) J. Chen and M. J. Cook, Org. Lett., 2013, 15, 1088 CrossRef PubMed; (k) T. D. Montgomery, Y. Zhu, N. Kagawa and V. H. Rawal, Org. Lett., 2013, 15, 1140 CrossRef CAS PubMed; (l) Y.-Q. Zhang, Y.-A. Yuan, G.-S. Liu and H. Xu, Org. Lett., 2013, 15, 3910 CrossRef CAS PubMed; (m) M. E. Kieffer, K. V. Chuang and S. E. Reisman, J. Am. Chem. Soc., 2013, 135, 5557 CrossRef CAS PubMed; (n) J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6802 CrossRef CAS PubMed; (o) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851 CrossRef CAS PubMed; (p) M.-C. Tong, X. Chen, J. Li, R. Huang, H. Tao and C.-J. Wang, Angew. Chem., Int. Ed., 2014, 53, 4680 CrossRef CAS PubMed.
- For selected examples, see: (a) C. Liu, W. Zhang, L.-X. Dai and S.-L. You, Org. Lett., 2012, 14, 4525 CrossRef CAS PubMed [Corrections: Org. Lett. 2012, 14, 5168]; (b) Q. Cai, C. Liu, X.-W. Liang and S.-L. You, Org. Lett., 2012, 14, 4588 CrossRef CAS PubMed; (c) X. Zhang, Z.-P. Yang, C. Liu and S.-L. You, Chem. Sci., 2013, 4, 3239 RSC; (d) Q. Cai, Q. Yin and S.-L. You, Asian J. Org. Chem., 2014, 3, 408 CrossRef CAS; (e) X. Zhang, L. Han and S.-L. You, Chem. Sci., 2014, 5, 1059 RSC; (f) L. Han, C. Liu, W. Zhang, X.-X. Shi and S.-L. You, Chem. Commun., 2014, 50, 1231 RSC; (g) D.-H. Duan, Q. Yin, S.-G. Wang and S.-L. You, Acta Chim. Sin., 2014, 72, 1001 CrossRef; (h) Q. Yin and S.-L. You, Org. Lett., 2014, 16, 2426 CrossRef CAS PubMed.
- (a) A. Gualandi, E. Emer, M. G. Capdevila and P. G. Cozzi, Angew. Chem., Int. Ed., 2011, 50, 7842 CrossRef CAS PubMed; (b) A. Gualandi, D. Petruzziello, E. Emer and P. G. Cozzi, Chem. Commun., 2012, 48, 3614 RSC.
- For an early preparation of C3-methyl substituted pyrrolodindolines from tryptamines, see: M. Kawahra, A. Nishida and M. B. Nakagawa, Org. Lett., 2000, 2, 675 CrossRef PubMed.
- When methyl (2-(1H-indol-3-yl)ethyl)carbamate was employed as a substrate, a dearomatized product with an indole-N substituted by dithiane was isolated (60% yield).
- For details, see the ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and analysis data for new compounds. CCDC 1041140. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc00780a |
| This journal is © The Royal Society of Chemistry 2015 |