Open Access Article
Open Access ArticleDibromoindium(III) cations as a π-Lewis acid: characterization of [IPr·InBr2][SbF6] and its catalytic activity towards alkynes and alkenes†‡
Bastien
Michelet
,
Jean-Rémy
Colard-Itté
,
Guillaume
Thiery
,
Régis
Guillot
,
Christophe
Bour
* and
Vincent
Gandon
*
Université Paris-Sud, ICMMO (UMR CNRS 8182), LabEx CHARM3AT, 91405 Orsay cedex, France. E-mail: vincent.gandon@u-psud.fr; Web: http://www.polycata.u-psud.fr Fax: + 33-169154747; Tel: + 33-169153931
First published on 24th March 2015
Abstract
[IPr·InBr2][SbF6] (2) (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) has been synthesized and characterized in the solid state. This complex proved to be a very active catalyst for hydroarylations, transfer hydrogenations, and cycloisomerizations.
Indium(III) salts of type InX3 (X = Cl, Br, I, OTf, NTf2) are enjoying increasing use in homogeneous molecular organic catalysis.1 In particular, Corey et al. showed that InBr3 and InI3 have strong affinity for alkynes and trigger elegant cationic polycyclizations.2 In a recent paper, this team reported that a catalytic mixture of InI3 and AgY (10 mol% each, Y = SbF6 or B[C6H3-3,5-(CF3)2]4) was an even more active initiator for such cyclizations than InI3 or InBr3.3,4 These two components are supposed to give rise to InX2+ cations, yet no analysis could be performed on the weakly soluble material, and the putative [InX2][Y] species could not be separated from AgX. The higher activity of InX2+ has been explained by the participation of the two vacant p-orbitals of indium in the coordination of the orthogonal π-orbitals of the alkyne cascade initiator in a crisscrossed geometry. On the other hand, we have shown that complexes of type L·GaCl2+, where L is a N-heterocyclic carbene (NHC), are more active than GaCl3 towards alkynes, even though they display only one vacant orbital.5 We reasoned that the synthesis and characterization of a well-defined indium complex of the (NHC)·InX2+ series and the evaluation of its π-acidity would shed light on that matter and advance the field for additional reasons: firstly, only a few indium(III) complexes of type (κ1-L)n·InX2+ displaying monocoordinating ligands have been characterized in the solid state.6 Most compounds actually display κ2-L bidentate ligands and are 8- or 12-electron species of type (κ2-L)·InX2+ or (κ2-L)2·InX2+.7 To the best of our knowledge, none of them have found applications in molecular catalysis. On the other hand, a 6-electron complex of type (κ1-L)·InX2+ should display interesting Lewis acid properties due to its unsaturated character. We are not aware of any such compound characterized in the solid state. Secondly, the formation of a donor–acceptor adduct between a NHC and a main group Lewis acid can give rise to easy-to-handle species. For instance, whereas GaCl3 is highly hygroscopic, [IPr·GaCl3] (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) is bench-stable.8 In that respect, it would be particularly interesting to use [IPr·InBr3] (1) instead of the hygroscopic InBr3 Lewis acid.9 This air-stable species is one of the very few indium complexes supported by NHC ligands.10 If the bromide abstraction can be achieved and provide a well-defined species, a unique opportunity to study the behavior of [L·InBr2]+ cations as a π-Lewis acid would be at hand.
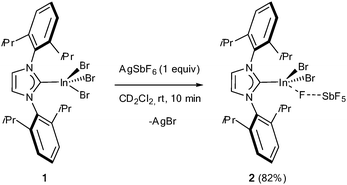
Addition of one equiv. of AgSbF6 to [IPr·InBr3] (1) in CD2Cl2 at room temperature led to the immediate formation of a grey precipitate (Scheme 1). Energy dispersive spectroscopy was used to qualitatively analyze the chemical composition of this precipitate, which reveals mainly the presence of AgBr. All 1H signals of the carbene moiety were found to be shifted, notably the signal corresponding to the olefinic backbone of the imidazolium ring, which was found at 7.60 ppm after the addition of the silver salt instead of 7.35 ppm for 1. The 19F NMR spectra showed a broad signal at −123.6 ppm, which is typical of a fluorine-bridged [M⋯F⋯SbF5] fragment in which each fluorine undergoes rapid exchange.5f,11 Thus, this reaction is likely to provide the desired complex 2 of formula [IPr·InBr2][SbF6], which could also be described as the fluorine-bridged [IPr·InBr2(μ-F)SbF5].
After filtration of the precipitate and slow evaporation, colorless crystals suitable for X-ray diffraction were collected (82% isolated yield). X-ray structure analysis confirmed the general structural assignment (Fig. 1).12 In 2, the In1–Br bond distances are significantly shorter than in 1 (2.4379(5) Å (av) vs. 2.496(2) Å (av)). The same is true for the In1–C1 bond (2.178(2) Å vs. 2.204(8) Å (av)). The In1–F1 distance (2.189(2) Å) is quite long for a standard In–F bond (expected around 2.05 Å).13 As a result of the sharing of F1 between In1 and Sb1, the Sb1–F1 bond is also longer than the other five Sb–F bonds (1.954(2) Å vs. 1.856(3) Å (av)). Thus, complex 1 represents a rare example of a perfluoroanion monocoordinated to a p-block metal,5f,14 and, as noted above, a rare example of a well-defined (κ1-L)·InX2+ complex.
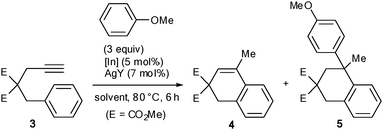
The catalytic activity of 2 as a π-Lewis acid was evaluated and compared to that of simple indium salts. The reaction of arenyne 3 with anisole was used as a prototype of a cationic cascade since it involves the activation of an alkyne to give 4 and then the activation of the alkene moiety of 4 to give the tandem product 5 (Table 1).15
| Entry | [In] | AgY | Solvent |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a (% conv.) 5a (% conv.) |
Yield of 5b (%) |
|---|---|---|---|---|---|
| a Determined by GC. b Isolated yields. c Trace amounts. d No reaction. e 4 is isolated in 75% yield. | |||||
| 1 | InCl3 | — | DCE | — | —c |
| 2 | InBr3 | — | DCE | 32/13 | 12 |
| 3 | InBr3 | AgSbF6 | DCE | 18/81 | 65 |
| 4 | 1 | — | DCE | — | —d |
| 5 | 1 | AgSbF6 | DCE | 20/80 | 62 |
| 6 | 2 | — | DCE | 29/71 | 68 |
| 7 | 1 | AgSbF6 | Toluene | 78/13 | 15e |
| 8 | 1 | AgSbF6 | THF | — | —d |
| 9 | 1 | AgBF4 | DCE | — | —d |
| 10 | 1 | AgPF6 | DCE | — | —d |
The reaction was first carried out in 1,2-dichloroethane (DCE) at 80 °C. With a catalytic amount of the poorly soluble InCl3, only trace amounts of products were monitored by gas chromatography (entry 1). With the soluble InBr3, product 5 was obtained as the minor component of the mixture and isolated only in 12% yield (entry 2). The joint use of InBr3 and AgSbF6 provided 5 as the major product in 65% isolated yield (entry 3).16 Complex 1 showed no catalytic activity (entry 4). On the other hand, a catalytic mixture of 1 and AgSbF6 gave similar results as InBr3/AgSbF6 (entry 5). It is worth noting that in the two-component reactions, the indium complex (InBr3 or 1) and AgSbF6 were premixed at room temperature for 5 min to ensure the formation of the corresponding cationic species (a precipitate forms instantly). Moreover, AgSbF6 shows no activity in this reaction. Cationic species 2 generated under these catalytic conditions was further evidenced by using it directly (entry 6). The products distribution and the isolated yield of 5 were found to be quite close to those of the two-component experiment. In toluene, the 1/AgSbF6 system was much less effective (entry 7) and in THF, no reaction took place (entry 8). Replacement of AgSbF6 by AgBF4 or AgPF6 was also detrimental to the reactivity (entries 9 and 10).
Complex 1 does not just represent a stable alternative to the hygroscopic InBr3. In some cases, the [IPr·InBr2]+ cation proved to be more active than InBr2+. When 1,2-dimethoxybenzene or 1-(phenylsulfonyl)indole were used instead of anisole in the transformation of 3, full conversion was reached faster and the isolated yields of 6 and 7 were better with the former (Table 2).
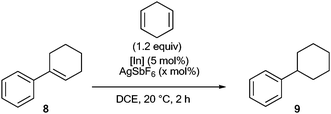
We next examined whether catalytic transfer hydrogenation of alkenes was possible (Table 3). The reduction of cyclohexenylbenzene 8 was attempted using 1,4-cyclohexadiene (1,4-CHD) as the hydrogen transfer agent.5e This reaction does not work with the neutral indium species InBr3 and 1 (entries 1 and 2), or with AgSbF6 alone. Yet it becomes very efficient with InBr2+ or [IPr·InBr2]+ (entries 3 and 4), both displaying similar activities.
Two other alkenes were also reduced using the in situ generated [IPr·InBr2]+ cation as the catalyst and 1,4-CHD as the hydrogen transfer agent (Scheme 2). For the transformation of the acyclic substrate 10, a temperature of 80 °C had to be applied. With the more reactive cyclic alkene 12, the reaction took place at rt.
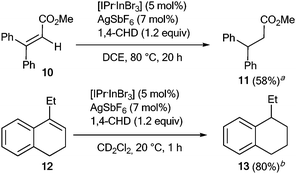 | ||
| Scheme 2 In(III)-catalyzed transfer hydrogenation of alkenes 10 and 12 (a isolated yield; b determined by 1H NMR using p-anisaldehyde as an internal standard). | ||
Hydrogenative cyclization of 3 in the presence of 1,4-CHD was also tested (Table 4). Again, the activity of the neutral indium species was marginal (entries 1 and 2). With InBr2+, the transformation required 6 h to reach completion at 80 °C and the isolated yield of 14 was 58% (entry 3). On the other hand, the reaction was over in 2 h with [IPr·InBr2]+ and the isolated yield was improved to 73% (entry 4).
Lastly, the cycloisomerization of the enyne 15 was attempted (Scheme 3).4c,17 When carried out with a catalytic mixture of [IPr·GaCl3] and AgSbF6 or [IPr·InBr3] and AgSbF6, full conversion was reached but the desired product 16 was obtained in low amounts and admixed with a complex mixture of unidentified products. Another cationizing agent was then tested, silver polyfluoro-tert-butyloxyaluminate [Ag][Al(pftb)4] (pftb = OC(CF3)3), which displays one of the most weakly coordinating anions known.18 While the joint use of [IPr·GaCl3] and [Ag][Al(pftb)4] still gave rise to decomposition, 16 could be obtained very cleanly with [IPr·InBr3] and [Ag][Al(pftb)4].19 This result shows that the NHC indium complex can surpass the gallium one and reveals an interesting counterion effect in this chemistry.
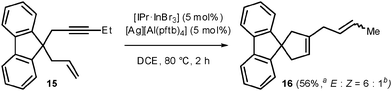 | ||
| Scheme 3 In(III)-catalyzed cycloisomerization of enyne 15 (a isolated yield; b determined by GLC analysis). | ||
In conclusion, this work demonstrates that the hygroscopic InBr3 salt can be advantageously replaced by the bench-stable [IPr·InBr3] complex to generate the catalytically active [IPr·InBr2]+ cation upon treatment with AgSbF6. This species, which is a rare type of cationic (κ1-L)·InX2+ complex, has been characterized in the solid state and proved to be a powerful π-Lewis acid. In various cases, we could show its superior activity compared to InBr2+, even with alkyne substrates, even though it displays a single vacant site. We also found one type of reaction for which [IPr·InBr2]+ proves to be more selective than the previously described [IPr·GaCl2]+, provided it is generated from [IPr·InBr3] and [Ag][Al(pftb)4] instead of AgSbF6. This interesting counterion effect is being explored in our laboratory.
This work was supported by UPS, CNRS, MESR, and IUF. We thank F. Brisset for EDS analysis.
Notes and references
- (a) C. G. Frost and J. P. Hartley, Mini-Rev. Org. Chem., 2004, 1, 1 CrossRef CAS; (b) T.-P. Loh and G.-L. Chua, Chem. Commun., 2006, 2739 RSC; (c) M. S. Singh and K. Raghuvanshi, Tetrahedron, 2012, 68, 8683 CrossRef CAS PubMed; (d) Y. Onishi, Y. Nishimoto, M. Yasuda and A. Baba, Org. Lett., 2014, 16, 1176 CrossRef CAS PubMed and the references therein.
- (a) K. Surendra, W. Qiu and E. J. Corey, J. Am. Chem. Soc., 2011, 133, 9724 CrossRef CAS PubMed; (b) W.-W. Qiu, K. Surendra, L. Yin and E. J. Corey, Org. Lett., 2011, 13, 5893 CrossRef CAS PubMed.
- K. Surendra and E. J. Corey, J. Am. Chem. Soc., 2014, 136, 10918 CrossRef CAS PubMed.
- For other examples of the joint use of catalytic amounts of InX3 and a silver salt of carbonyl-ene, Mukayama-aldol, and cycloisomerization reactions, see respectively: (a) J.-F. Zhao, T.-B. W. Tjan and T.-P. Loh, Tetrahedron Lett., 2010, 51, 5649 CrossRef CAS PubMed; (b) J.-F. Zhao, B.-H. Tan and T.-P. Loh, Chem. Sci., 2011, 2, 349 RSC; (c) L.-G. Zhuo, J.-J. Zhang and Z.-X. Yu, J. Org. Chem., 2012, 79, 8527 CrossRef PubMed.
- (a) H.-J. Li, R. Guillot and V. Gandon, J. Org. Chem., 2010, 75, 8435 CrossRef CAS PubMed; (b) S. Tang, J. Monot, A. El-Hellani, B. Michelet, R. Guillot, C. Bour and V. Gandon, Chem. – Eur. J., 2012, 18, 10239 CrossRef CAS PubMed; (c) A. El- Hellani, J. Monot, R. Guillot, C. Bour and V. Gandon, Inorg. Chem., 2013, 52, 506 CrossRef CAS PubMed; (d) A. El-Hellani, J. Monot, S. Tang, R. Guillot, C. Bour and V. Gandon, Inorg. Chem., 2013, 52, 11493 CrossRef CAS PubMed; (e) B. Michelet, C. Bour and V. Gandon, Chem. – Eur. J., 2014, 20, 14488 CrossRef CAS PubMed; (f) C. Bour, J. Monot, S. Tang, R. Guillot, J. Farjon and V. Gandon, Organometallics, 2014, 33, 594 CrossRef CAS; (g) C. Bour and V. Gandon, Coord. Chem. Rev., 2014, 279, 43 CrossRef CAS PubMed.
- We are aware of only two 12-electron InX2+ ions: (a) [InI2(Me2SO)4][InI4]: F. W. B. Einstein and D. G. Tuck, J. Chem. Soc. D, 1970, 1182 RSC; (b) [InCl2(Ph3PO)4][InCl4]: W. T. Robinson, C. J. Wilkins and Z. Zeying, J. Chem. Soc., Dalton Trans., 1990, 219 RSC.
- (a) S. Dagorne and D. A. Atwood, Chem. Rev., 2008, 108, 4037 CrossRef CAS PubMed; (b) M. Sigl, A. Schier and H. Schmidbaur, Z. Naturforsch., 1998, 53b, 1301 Search PubMed; (c) F. Cheng, S. I. Friend, A. L. Hector, W. Levason, G. Reid, M. Webster and W. Zhang, Inorg. Chem., 2008, 47, 9691 CrossRef CAS PubMed; (d) C. Gurnani, M. Jura, W. Levason, R. Ratnani, G. Reid and M. Webster, Dalton Trans., 2009, 1611 RSC.
- N. Marion, E. C. Escudo-Adán, J. Benet-Buchholz, E. D. Stevens, L. Fensterbank, M. Malacria and S. P. Nolan, Organometallics, 2007, 26, 3256 CrossRef CAS.
- R. J. Baker, A. J. Davies, C. Jones and M. Kloth, J. Organomet. Chem., 2002, 656, 203 CrossRef CAS.
- (a) S. J. Black, D. E. Hibbs, M. B. Hursthouse, C. Jones, K. M. Abdul Malik and N. A. Smithies, J. Chem. Soc., Dalton Trans., 1997, 4313 RSC; (b) C. D. Abernethy, M. L. Cole and C. Jones, Organometallics, 2000, 19, 4852 CrossRef CAS; (c) J. H. Cotgreave, D. Colclough, G. Kociok-Köhn, G. Ruggiero, C. G. Frost and A. S. Weller, Dalton Trans., 2004, 1519 RSC; (d) For a review, see: C. Fliedel, G. Schnee, T. Avilés and S. Dagorne, Coord. Chem. Rev., 2014, 275, 63 CrossRef CAS PubMed.
- (a) W. H. Hersh, J. Am. Chem. Soc., 1985, 107, 4599 CrossRef CAS; (b) R. V. Honeychuck and W. H. Hersh, Inorg. Chem., 1989, 28, 2869 CrossRef CAS.
- CCDC 1044808.
- V. Mastelaro, S. Ribeiro, Y. Messaddeq and M. Aeggerter, J. Mater. Sci., 1996, 31, 3441 CAS.
- (a) H. Grützmacher and H. Pritzkow, Organometallics, 1991, 10, 938 CrossRef; (b) J. M. Blackwell, W. E. Piers and R. McDonald, J. Am. Chem. Soc., 2002, 124, 1295 CrossRef CAS PubMed; (c) X. Zhang, R. Qiu, N. Tan, S. Yin, J. Xia, S. Luo and C.-T. Au, Tetrahedron Lett., 2010, 51, 153 CrossRef CAS PubMed.
- This reaction also works well with GaCl3 (see ref. 5a) or [IPr·GaCl2][SbF6] (see ref. 5b). On the other hand, the aluminium counterparts of these species are not active.
- Silver salts are not active in the reactions presented herein. The use of a slight excess of AgY compared to the indium complex is to ensure complete cationization. The yields are usually slightly better with a 5/7 ratio than with a 5/5 one.
- Y. Miyanahana and N. Chatani, Org. Lett., 2006, 8, 2155 CrossRef PubMed.
- I. Krossing, Chem. – Eur. J., 2001, 7, 490 CrossRef CAS.
- Neither [IPr·InBr3] nor [Ag][Al(pftb)4] alone are active in this reaction.
Footnotes |
| † Dedicated to Prof. A. M. Echavarren on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available: Synthetic procedures and crystallographic details for 2. CCDC 1044808. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc00740b |
| This journal is © The Royal Society of Chemistry 2015 |